��Ŀ����
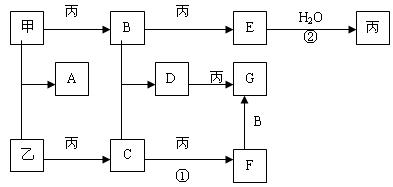
��12�֣�A��B��C����ѧ��ѧ�������������ʣ�����֮����ת����ϵ���£����ַ�Ӧ������������ȥ����

��1����A��һ�ֵ���ɫ���ʹ��壬��B�Ի���������IJ���Ӱ���� ����B��C�Ļ�ѧ����ʽΪ ��
��2����A��һ�ֽ�����C�ǵ���ɫ���壬��B�ĵ���ʽΪ ��C������ ˮ��Ӧ�Ļ�
ˮ��Ӧ�Ļ� ѧ����ʽΪ ��
ѧ����ʽΪ ��
��3����C�Ǻ���ɫ���壬��A�Ļ�ѧʽ����Ϊ ����д��C��ˮ��Ӧ�Ļ�ѧ���� ʽ ��
ʽ ��

��1����A��һ�ֵ���ɫ���ʹ��壬��B�Ի���������IJ���Ӱ���� ����B��C�Ļ�ѧ����ʽΪ ��
��2����A��һ�ֽ�����C�ǵ���ɫ���壬��B�ĵ���ʽΪ ��C������
 ˮ��Ӧ�Ļ�
ˮ��Ӧ�Ļ� ѧ����ʽΪ ��
ѧ����ʽΪ ����3����C�Ǻ���ɫ���壬��A�Ļ�ѧʽ����Ϊ ����д��C��ˮ��Ӧ�Ļ�ѧ����
 ʽ ��
ʽ ����1���γ����꣨2�֣� 2SO2+O2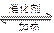 2SO3��2�֣�
2SO3��2�֣�
��2��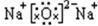 2Na2O2+2H2O=4NaOH+O2�� ����2�֣���4�֣�
2Na2O2+2H2O=4NaOH+O2�� ����2�֣���4�֣�
��3��N2��NH3 ��2�֣�д��һ�����ɣ���
3NO2+H2O==2HNO3+NO ��2�֣�
 2SO3��2�֣�
2SO3��2�֣���2��
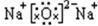 2Na2O2+2H2O=4NaOH+O2�� ����2�֣���4�֣�
2Na2O2+2H2O=4NaOH+O2�� ����2�֣���4�֣���3��N2��NH3 ��2�֣�д��һ�����ɣ���
3NO2+H2O==2HNO3+NO ��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ

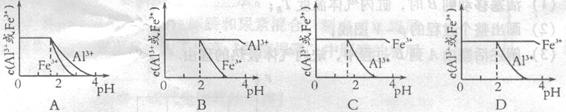
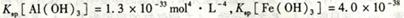 mol4��Lһ4������pH=0��Ũ�Ⱦ�Ϊ0��04mol��L-1��Al3+��Fe3+��Һ�м���A������������Ӧˮ�������Һ���Ե���pH(����Һ�������)���ù�����Al3+��Fe3+��Ũ����pH��ϵ��ȷ����
mol4��Lһ4������pH=0��Ũ�Ⱦ�Ϊ0��04mol��L-1��Al3+��Fe3+��Һ�м���A������������Ӧˮ�������Һ���Ե���pH(����Һ�������)���ù�����Al3+��Fe3+��Ũ����pH��ϵ��ȷ����