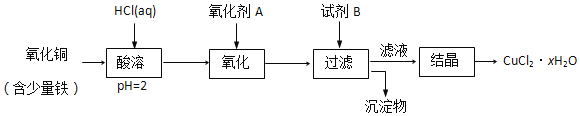
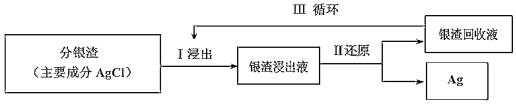
Ő‚ńŅńŕ»›
°ĺŐ‚ńŅ°ŅA°ĘB°ĘC°ĘDĺýő™÷–—߼Į—ß≥£ľŻĶńīŅĺĽőÔ£¨A «Ķ•÷ °£ňŁ√«÷ģľš”–»ÁŌ¬Ķń∑ī”¶ĻōŌĶ£ļ
£®1£©»ŰA «Ķ≠Ľ∆…ęĻŐŐŚ£¨C°ĘD «—űĽĮőÔ£¨C «‘ž≥…ňŠ”ÍĶń÷ų“™őÔ÷ £¨ĶęC“≤”–∆šĻ„∑ļĶń”√Õĺ£¨–ī≥Ų∆š÷–Ķń2łŲ”√Õĺ£ļ__________________________°£
£®2£©»ŰB «∆ÝŐ¨«‚ĽĮőÔ£¨C°ĘD «—űĽĮőÔ«“ĽŠ‘ž≥…Ļ‚ĽĮ—ß—ŐőŪőŘ»ĺ°£B”ŽC‘ŕ“Ľ∂®ŐűľĢŌ¬∑ī”¶…ķ≥…ĶńA «īů∆ÝĶń÷ų“™≥…∑÷£¨–ī≥Ųł√∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺ£ļ________________°£
£®3£©»ŰDőÔ÷ ĺŖ”–ŃĹ–‘£¨Ęŕ°ĘĘŘ∑ī”¶ĺý“™”√«ŅľÓ»‹“ļ°£»ÁĘ‹∑ī”¶ ĪÕ®»ŽĻżŃŅĶń“Ľ÷÷“ż∆ūő¬ “–ß”¶Ķń÷ų“™∆ÝŐŚ£¨–ī≥Ųł√∆ÝŐŚĶńĶÁ◊” Ĺ£ļ_________£¨AĶń‘™ňō‘ŕ÷‹∆ŕĪŪ÷–ĶńőĽ÷√£ļ__________________°£
£®4£©»ŰA «Őę—Űń‹ĶÁ≥ō”√ĶńĻ‚∑Ł≤ńŃŌ°£C°ĘDő™ń∆—ő£¨ŃĹ÷÷őÔ÷ ÷–ń∆°Ę—űÕ‚Ķń‘™ňōő™Õ¨“Ľ÷ų◊Ś£¨«“»‹“ļĺýŌ‘ľÓ–‘°£–ī≥ŲĘŕ∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺ£ļ_____________________°£DĶńĽĮ—ß Ĺ «______°£
°ĺīūįł°Ņ∆Įį◊°Ę…Īĺķ°ĘŌŻ∂ĺ°Ę◊ųő™ŃÚňŠĶń‘≠ŃŌĶ» 4NH3£ę6NO![]() 5N2£ę6H2O Ķ໿÷‹∆ŕĘůA◊Ś
5N2£ę6H2O Ķ໿÷‹∆ŕĘůA◊Ś ![]() Si£ę2NaOH£ęH2O=Na2SiO3£ę2H2°Ł Na2CO3
Si£ę2NaOH£ęH2O=Na2SiO3£ę2H2°Ł Na2CO3
°ĺĹ‚őŲ°Ņ
£®1£©Ķ≠Ľ∆…ęĶńĻŐŐŚĶ•÷ «ŃÚ£¨B «H2S£ĽCő™SO2£¨Dő™SO3£Ľ
£®2£© »ŰB «∆ÝŐ¨«‚ĽĮőÔ£¨C°ĘD «—űĽĮőÔ«“ĽŠ‘ž≥…Ļ‚ĽĮ—ß—ŐőŪőŘ»ĺ°£B”ŽC‘ŕ“Ľ∂®ŐűľĢŌ¬∑ī”¶…ķ≥…ĶńA «īů∆ÝĶń÷ų“™≥…∑÷£¨‘ÚA°ĘB°ĘC°ĘD∑÷Īūő™N2°ĘNH3°ĘNO°ĘNO2£Ľ
£®3£©÷–—ßĹ◊∂ő—ßŌįĶńŃĹ–‘őÔ÷ ”–¬Ńľį¬ŃĶń—űĽĮőÔļÕ«‚—űĽĮőÔ£¨łýĺ›őÔ÷ ÷ģľšĶń◊™ĽĮĻōŌĶŅ…÷™£ļA «Al£ĽB «Al2O3£¨C «NaAlO2£¨D «Al(OH)3£Ľ
£®4£©»ŰA «Őę—Űń‹ĶÁ≥ō”√ĶńĻ‚∑Ł≤ńŃŌ£¨‘ÚA «ĺßŐŚSi£ĽC°ĘDő™ń∆—ő£¨ŃĹ÷÷őÔ÷ ÷–ń∆°Ę—űÕ‚Ķń‘™ňōő™Õ¨“Ľ÷ų◊Ś£¨«“»‹“ļĺýŌ‘ľÓ–‘°£łýĺ›őÔ÷ Ķń◊™ĽĮĻōŌĶľį“—÷™ŐűľĢŅ…÷™B «SiO2£ĽC «Na2SiO3£ĽD «Na2CO3°£
£®1£©”…“‘…Ō∑÷őŲŅ…÷™£¨A «ŃÚ£¨B «H2S£ĽCő™SO2£¨Dő™SO3£¨SO2Ķń”√Õĺ»Á∆Įį◊°Ę…Īĺķ£¨÷∆ĪłŃÚňŠĶ»°£
£®2£© ”…“‘…Ō∑÷őŲŅ…÷™£¨A°ĘB°ĘC°ĘD∑÷Īūő™N2°ĘNH3°ĘNO°ĘNO2£¨B”ŽC‘ŕ“Ľ∂®ŐűľĢŌ¬∑Ę…ķ∑ī”¶4NH3+6NO![]() 5N2+6H2O «Ļť÷–∑ī”¶£¨≤ķ…ķĶ™∆Ý°£
5N2+6H2O «Ļť÷–∑ī”¶£¨≤ķ…ķĶ™∆Ý°£
£®3£©”…“‘…Ō∑÷őŲŅ…÷™£¨A «Al£ĽB «Al2O3£¨C «NaAlO2£¨D «Al(OH)3°£Õ®»ŽĶńĶľ÷¬ő¬ “–ß”¶Ķń∆ÝŐŚ «CO2£¨∆šĶÁ◊” Ĺ «![]() £Ľ¬Ń‘™ňōĶńőĽ÷√ő™Ķ໿÷‹∆ŕĘůA◊Ś£Ľ
£Ľ¬Ń‘™ňōĶńőĽ÷√ő™Ķ໿÷‹∆ŕĘůA◊Ś£Ľ
£®4£©”…“‘…Ō∑÷őŲŅ…÷™£¨A «ĺßŐŚSi£¨ B «SiO2£¨C «Na2SiO3£¨D «Na2CO3°£Ęŕ∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺ£ļSi£ę2NaOH£ęH2O=Na2SiO3£ę2H2°Ł°£DĶńĽĮ—ß Ĺ «Na2CO3°£