��Ŀ����
ij�о���ѧϰС��Ϊ��̽������ĵ������������������ʵ�顣
̽��Ũ�ȶԴ������̶ȵ�Ӱ��
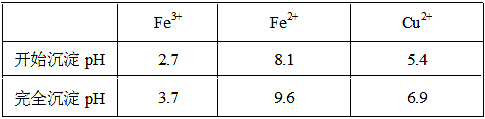
��pH�Ʋⶨ25��ʱ��ͬŨ�ȵĴ����pH���������£�
�ش��������⣺
��1��д������ĵ��뷽��ʽ�� ��
��2��������Һ�д��ڵ����� ��
��3�����ݱ������ݣ����Եó�������������ʵĽ��ۣ�����Ϊ�ó��˽��۵������� ��
��4���ӱ��е����ݣ������Եó���һ���ۣ����Ŵ���Ũ�ȵļ�С������ĵ���̶ȣ�������С�䣩 ��
̽��Ũ�ȶԴ������̶ȵ�Ӱ��
��pH�Ʋⶨ25��ʱ��ͬŨ�ȵĴ����pH���������£�
| ����Ũ��(mol/L) | 0.0010 | 0.0100 | 0.0200 | 0.1000 | 0.2000 |
| pH | 3.88 | 3.38 | 3.23 | 2.88 | 2.73 |
�ش��������⣺
��1��д������ĵ��뷽��ʽ�� ��
��2��������Һ�д��ڵ����� ��
��3�����ݱ������ݣ����Եó�������������ʵĽ��ۣ�����Ϊ�ó��˽��۵������� ��
��4���ӱ��е����ݣ������Եó���һ���ۣ����Ŵ���Ũ�ȵļ�С������ĵ���̶ȣ�������С�䣩 ��
��1��CH3COOH  CH3COO- + H+ ��2�֣�
CH3COO- + H+ ��2�֣�
��2��H2O, CH3COOH, CH3COO- , H+ , OH- ��3��,��һ����1�֣���
��3��������Ũ��Ϊ0.001mol/L ʱ����Һ�е�������Ũ��С��0.001mol/L��3�֣��������������ɣ�
��4������2�֣�
 CH3COO- + H+ ��2�֣�
CH3COO- + H+ ��2�֣���2��H2O, CH3COOH, CH3COO- , H+ , OH- ��3��,��һ����1�֣���
��3��������Ũ��Ϊ0.001mol/L ʱ����Һ�е�������Ũ��С��0.001mol/L��3�֣��������������ɣ�
��4������2�֣�
���������
��1��CH3COOH��������ʣ� CH3COOH
 CH3COO- + H+
CH3COO- + H+ ��2��������Һ�д��ڴ����˯�ò��ֵ��룬���Դ�����������H2O, CH3COOH, CH3COO- , H+ , OH-
��3��������Ũ��Ϊ0.001mol/L ʱ����Һ�е�������Ũ��С��0.001mol/L���������ֵ��룬Ϊ������ʡ�
��4������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 H++SO42-��
H++SO42-�� ���䣻��OH�C��Ŀ����H+��Ŀ��С����pH���� c(H+)��c(OH�C)�ij˻���С
���䣻��OH�C��Ŀ����H+��Ŀ��С����pH���� c(H+)��c(OH�C)�ij˻���С H����SO42-
H����SO42- H����SO42
H����SO42