��Ŀ����
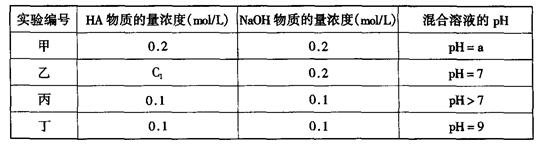
��11�֣������£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
 ��ش�
��ش�
(1)�������������ʵ���������Ӽ�����������������a (�����Һ��pH)��˵��HA��ǿ�ỹ���� �� ��
(2)�������������ʵ�����������������������C1�Ƿ�һ������0.2mol��L (ѡ��ǡ���)�������Һ������Ũ��c(A��)��c(Na+)�Ĵ�С��ϵ��
(3)�ӱ���ʵ����������HA�� ��(ѡ�ǿ��������)��
�û����Һ������Ũ���ɴ�С��˳����
(4)����ʵ�����û����Һ����ˮ�������c(OH��)�� mol��L
д���û����Һ��������ʽ�ľ�ȷ���(���������Ƽ��㣬�ش�ȷֵ�������һ��Ҫ����)�� c(Na+)��c(A��)�� mol��L
 ��ش�
��ش�(1)�������������ʵ���������Ӽ�����������������a (�����Һ��pH)��˵��HA��ǿ�ỹ���� �� ��
(2)�������������ʵ�����������������������C1�Ƿ�һ������0.2mol��L (ѡ��ǡ���)�������Һ������Ũ��c(A��)��c(Na+)�Ĵ�С��ϵ��
| A��ǰ�ߴ� | B�����ߴ� | C��������� | D�����ж� |
�û����Һ������Ũ���ɴ�С��˳����
(4)����ʵ�����û����Һ����ˮ�������c(OH��)�� mol��L
д���û����Һ��������ʽ�ľ�ȷ���(���������Ƽ��㣬�ش�ȷֵ�������һ��Ҫ����)�� c(Na+)��c(A��)�� mol��L
��1��a=7ʱ��HA��ǿ�ᣬa��7ʱ��HA������
��2����һ���� c
��3���� c(Na+)��c(A-)��c(OH-)��c(H+)
��4��10-5 10-5-10-9
��2����һ���� c
��3���� c(Na+)��c(A-)��c(OH-)��c(H+)
��4��10-5 10-5-10-9
���������(1)�������������ʵ���������Ӽ��������������Ϊ�ǵ������Ũ�Ȼ�ϣ�HA��ǿ�ᣬ��������ǿ��ǿ���Σ�a=7��HA�����ᣬ��������ǿ�������Σ�ˮ���Լ��ԣ�a��7������a=7ʱ��HA��ǿ�ᣬa��7ʱ��HA�����ᡣ
(2)�������������ʵ�������������������������Ϊ������PH=7�������е���غ�ɵû����Һ������Ũ��c(A��)=c(Na+)��C1��һ������0.2mol��L��
(3)�ӱ���ʵ����������HA�����ᣬ��Ϊ�ǵ������Ũ�Ȼ�ϣ�a��7��������ǿ�������Σ�ˮ���Լ��ԣ�����HA�����ᡣ�û����Һ������Ũ���ɴ�С��˳���� c(Na+)��c(A-)��c(OH-)��c(H+)��
��4������ʵ�����û����ҺPH=9����ˮ�������c(OH��)��10-5 mol��L ��Kw=10-14
c(H+)��10-9 mol��L �ɵ���غ�c��Na+��+c��H+�� =c��OH_��+c��A-��,c(OH��)-c��H+��= c(Na+)��c(A��) ����c(Na+)��c(A��)��10-5-10-9��
����������ϼ���ʱҪע�⣺�������Ũ�ȵ�ǿ��ǿ���ϣ������ԣ��������Ũ�ȵ�ǿ��������ʱ���������ɵ���ǿ�������Σ�������Һ�����ԣ��������Ũ�ȵ�ǿ��������ʱ���������ɵ���ǿ�������Σ�������Һ�Լ��ԡ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
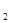 ����Һ�У����и�������һ�����ܴ����������
����Һ�У����и�������һ�����ܴ����������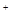 ��Fe
��Fe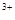 ��SO
��SO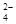 ��Cl
��Cl
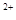 ��Mg
��Mg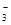 ��NO
��NO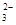
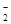 ��OH
��OH