��Ŀ����
���Ṥҵβ���ж�������ĺ�������0.05%(�������)ʱ�辭����������ŷš�ijУ��ѧ��ȤС�����ⶨij���Ṥ���ŷ�β���ж�������ĺ������ֱ�������·�����
[����]������ͼ��ʾ��ͼ������������B����ȷ����ͨ����β���������β��ͨ��һ�������֪Ũ�ȵĵ�ˮ�вⶨSO2�ĺ�������ϴ��ƿC����Һ��ɫ��ʧʱ�������رջ���A��
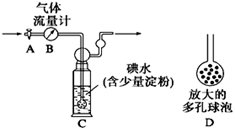
��1��ϴ��ƿC�е���ĩ������һ���������D���������ʵ���ȷ�ȣ���������___________________________________��
��2��ϴ��ƿC����Һ��ɫ��ʧ��û�м�ʱ�رջ���A����õ�SO2����_________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
[�ҷ���]��ʵ�鲽������������ͼ��ʾ��
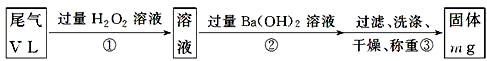
��3����������H2O2��������
��4��д��������з�Ӧ�Ļ�ѧ����ʽ______________________________________
��5���������Ba(OH)2�Ƿ��������жϷ�����_______________________________
___________________________________________________________________��
��6��ͨ����β�����ΪV L(�ѻ���ɱ�״��)ʱ����β���ж�������ĺ���(�������)Ϊ_____________________________(�ú���V��m�Ĵ���ʽ��ʾ)��
[����]������ͼ��ʾ��ͼ������������B����ȷ����ͨ����β���������β��ͨ��һ�������֪Ũ�ȵĵ�ˮ�вⶨSO2�ĺ�������ϴ��ƿC����Һ��ɫ��ʧʱ�������رջ���A��

��1��ϴ��ƿC�е���ĩ������һ���������D���������ʵ���ȷ�ȣ���������___________________________________��
��2��ϴ��ƿC����Һ��ɫ��ʧ��û�м�ʱ�رջ���A����õ�SO2����_________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
[�ҷ���]��ʵ�鲽������������ͼ��ʾ��
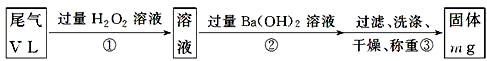
��3����������H2O2��������
��4��д��������з�Ӧ�Ļ�ѧ����ʽ______________________________________
��5���������Ba(OH)2�Ƿ��������жϷ�����_______________________________
___________________________________________________________________��
��6��ͨ����β�����ΪV L(�ѻ���ɱ�״��)ʱ����β���ж�������ĺ���(�������)Ϊ_____________________________(�ú���V��m�Ĵ���ʽ��ʾ)��
��1��������������Һ�ĽӴ������������SO2���ˮ��Ӧ
��2��ƫ��
��3��ʹSO2��ȫת��ΪSO42-
��4��H2SO4+Ba(OH)2=BaSO4��+2H2O
��5�����÷ֲ�����ϲ���Һ�м����μ�Ba(OH)2��Һ�������������������Ba(OH)2����������֮���㡣
��6��22.4m/233V��2240m/233V% (3��)
��2��ƫ��
��3��ʹSO2��ȫת��ΪSO42-
��4��H2SO4+Ba(OH)2=BaSO4��+2H2O
��5�����÷ֲ�����ϲ���Һ�м����μ�Ba(OH)2��Һ�������������������Ba(OH)2����������֮���㡣
��6��22.4m/233V��2240m/233V% (3��)
����������������õ�ԭ��Ϊ��SO2+I2+2H2O=H2SO4+2HI�����ⶨʣ������������������β���ж�������ĺ�����
��1��ϴ��ƿC�е���ĩ������һ���������D����������SO2���ˮ�ĽӴ������ʹSO2�͵�ˮ��ַ�Ӧ��
�ʴ�Ϊ������SO2���ˮ�ĽӴ������ʹSO2�͵�ˮ��ַ�Ӧ��
��2��ϴ��ƿC����Һ��ɫ��ʧ��û�м�ʱ�رջ���A����ͨ��β��������������SO2����ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
�ҷ������õ�ԭ��Ϊ��SO2+H2O2=H2SO4��H2SO4+Ba��OH��2=BaSO4��+2H2O�����������ᱵ����������β���ж����������������������β��������������
��3���ɷ���ԭ����֪��H2O2�ǽ�SO2��ȫת��ΪSO42-��
�ʴ�Ϊ����SO2��ȫת��ΪSO42-��
��4��������з�Ӧ�Ļ�ѧ����ʽΪ��H2SO4+Ba��OH��2=BaSO4��+2H2O��
�ʴ�Ϊ��H2SO4+Ba��OH��2=BaSO4��+2H2O��
��5���������Ba��OH��2�Ƿ��������жϷ����ǣ����÷ֲ�����ϲ���Һ�м����μ�Ba��OH��2��Һ���������������˵��Ba��OH��2�����������㣻
�ʴ�Ϊ�����÷ֲ�����ϲ���Һ�м����μ�Ba��OH��2��Һ���������������˵��Ba��OH��2�����������㣻
��6��mg�����ᱵ�����������ᱵ�����ʵ���Ϊmg��233g/mol= (m/233)mol��������Ԫ���غ��֪������������Ϊ(m/233)mol��22.4L/mol="(22.4m/233)" L����β���ж���������������(22.4m/233) L/ VL=22.4m/233V���ʴ�Ϊ��22.4m/233V��
���������⿼��ѧ����ʵ��ԭ����ʵ����������⡢ʵ�鷽����ơ�Ԫ�ػ��������ʡ���ѧ����ȣ��Ѷ��еȣ����ʵ��ԭ���ǽ���Ĺؼ�����Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢������������

��ϰ��ϵ�д�
�����Ŀ