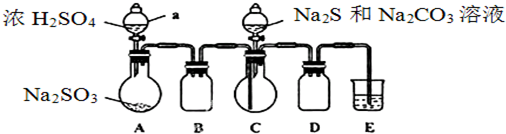
��Ŀ����
8���ڱ�״���£���aLNH3��ȫ����ˮ�õ�VmL��ˮ����Һ���ܶ�Ϊ��g��cm-3�����ʵ���������Ϊ�أ����ʵ����ʵ���Ũ��ΪC mol/L��������������ȷ������1�����ð�ˮ�����ʵ���Ũ��C���Ա�ʾΪ$\frac{1000a}{22.4V}$��$\frac{1000�Ѧ�}{17}$�����軯��
��2��������Һ�м���VmLˮ��������Һ����������С��0.5�أ�����ڣ�С�ڣ�����ڣ�
��3��������Һ�е�������ˮ��������Һ�����ʵ���Ũ�ȴ���0.5C������ڣ�С�ڣ�����ڣ�
���� ��1������n=$\frac{V}{{V}_{m}}$���㰱�����ʵ������ٸ���c=$\frac{n}{V}$�������ʵ���Ũ�ȣ����߸���c=$\frac{1000�Ѧ�}{M}$�������ʵ���Ũ�ȣ�
��2��ˮ���ܶȱȰ�ˮ���ܶȴ��������İ�ˮ��ˮ��ˮ�������������Ϻ���Һ����������ԭ��ˮ��2��������Һ�а�����������ͬ��
��3����Ϻ���Һ����Ϊԭ��ˮ������2��������ҺŨ�ȱ�ϡ��ϡ��ˮ���ܶȸ��ʻ�Ϻ���Һ���С��ԭ��ˮ�����2��������Һ�а��������ʵ������䣮
��� �⣺��1���������ʵ���Ϊ$\frac{aL}{22.4L/mol}$=$\frac{a}{22.4}$mol���ʰ�ˮ�����ʵ���Ũ��Ϊ$\frac{\frac{a}{22.4}mol}{V��1{0}^{-3}L}$=$\frac{1000a}{22.4V}$mol/L������c=$\frac{1000�Ѧ�}{M}$��֪���ð�ˮ�����ʵ���Ũ��Ϊ$\frac{1000�Ѧ�}{17}$mol/L��
�ʴ�Ϊ��$\frac{1000a}{22.4V}$��$\frac{1000�Ѧ�}{17}$��
��2��ˮ���ܶȱȰ�ˮ���ܶȴ��������İ�ˮ��ˮ��ˮ�������������Ϻ���Һ����������ԭ��ˮ��2������Һ�а�����������ͬ����������������Һ���ʵ���������С��0.5�أ�
�ʴ�Ϊ��С�ڣ�
��3����Ϻ���Һ����Ϊԭ��ˮ������2��������ҺŨ�ȱ�ϡ��ϡ��ˮ���ܶȸ��ʻ�Ϻ���Һ���С��ԭ��ˮ�����2��������Һ�а��������ʵ������䣬��������Ϻ�������Һ�����ʵ���Ũ�ȴ���0.5C��
�ʴ�Ϊ�����ڣ�
���� ���⿼�����ʵ���Ũ�ȡ����������ļ��㣬��������ǽ���Ĺؼ������������������������ʵ���Ũ�ȹ�ϵ��ע�ⰱˮ��Ũ��Խ���ܶ�ԽС��

| A�� | Cu2+��Fe2+ | B�� | Cu2+��Fe3+ | C�� | Fe2+��Fe3+ | D�� | Fe2+��Cl- |
| A�� | 1��1��1 | B�� | 1��2��1 | C�� | 1��1��2 | D�� | 4��3��2 |
| A�� | KNO3 | B�� | ZnSO4 | C�� | KCl | D�� | FeSO4 |
| A�� | ��ȩ | B�� | ����ȩ��ͬϵ�� | ||
| C�� | CH3CH2COCH3 ��2-��ͪ�� | D�� | CH2=C��CH3��CHO |
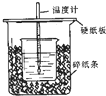 ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺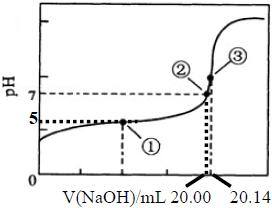 �������ճ������г��������ᣮ
�������ճ������г��������ᣮ