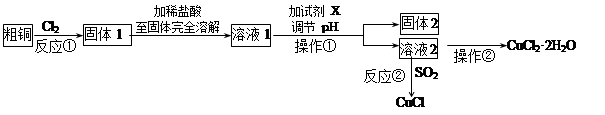��Ŀ����
��2014��ɽ��ʡɽ���и�����ѧ�ڵ�һ���¿���ѧ�Ծ���
����ʵ���У�Ϊʵ��ʵ��Ŀ�Ķ�������ӵ��ǣ�
A��ֻ�Т٢ڢ� B��ֻ�Т٢ڢ� C��ֻ�Тڢۢ� D���٢ڢۢ�
����ʵ���У�Ϊʵ��ʵ��Ŀ�Ķ�������ӵ��ǣ�
| | ʵ�� | �����Լ� | ʵ��Ŀ�� |
| �� | ��ʯ��ˮ��Ӧ | CuSO4��Һ | ��KMnO4������Һ������Ȳ�Ļ�ԭ�� |
| �� | CH3CH2Br��NaOH��Һ���� | HNO3��Һ | ��AgNO3��Һ����CH3CH2Br�е�Br |
| �� | ������ϡH2SO4ˮԡ���� | NaOH��Һ | ��������Һ����ˮ�����Ļ�ԭ�� |
| �� | C2H5OH��ŨH2SO4������170 �� | NaOH��Һ | ��Br2��CCl4��Һ֤���÷�ӦΪ��ȥ��Ӧ |
D
�ٵ�ʯ��ˮ��Ӧ������Ȳ���������⣬��������ͭ�ɳ��ӣ�Ȼ����KMnO4������Һ������Ȳ�Ļ�ԭ�ԣ�ʵ�����������ȷ����CH3CH2Br��NaOH��Һ���ȣ�����ˮ�ⷴӦ�������������ԣ����������������飬ʵ�����������ȷ���۵�����ϡH2SO4ˮԡ���ȣ�����ˮ�����������ǣ�����������Ӧ�ڼ��������£�û���ڼ��������£�ʵ�鲻�������ʴ���C2H5OH��ŨH2SO4������170�棬������ȥ��Ӧ������ϩ��NaOH��Һ�ɳ��ӣ���ϩʹ���������Һ��ɫ��ʵ�����������ȷ���ݱ���Һ�巴Ӧ�������屽��HBr��Ȼ������Ȼ�̼������ȡ�����ϲ���Һ������������dz��ɫ������ʵ�����������ȷ����ѡD��

��ϰ��ϵ�д�
�����Ŀ
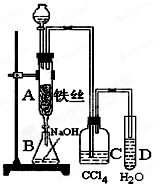
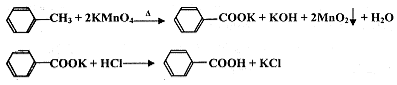
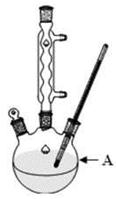

 �й��������↑ʼ�����ͳ�����ȫ��pH���±���
�й��������↑ʼ�����ͳ�����ȫ��pH���±���