ћвƒњƒЏ»Ё
°Њћвƒњ°њ“—÷™ґю‘™ЋбH2X“„»№”ЏЋЃ
£®1£©≥£ќ¬ѕ¬£ђѕт0.1mol/LµƒЋб љ—ќNaHXµƒ»№“Ї÷–µќ»лЉЄµќ„ѕ…Ђ ѓ»п ‘“Ї£ђ»№“Ї±д≥…Їм…Ђ°£
Ґў»ф≤вµ√іЋ»№“ЇµƒpH=1£ђ‘тNaHX»№”ЏЋЃ ±µƒµзјлЈљ≥ћ љќ™__________
ҐЏ»фNaHX»№“Ї÷–ƒ№Љм≤вµљH2XЈ÷„”£ђ‘т»№“Ї÷–c(X2-)__________£®ћо>°Ґ<їт=£©c(H2X)£ђc(X2-)+c(HX-)+c(H2X)=__________mol/L
£®2£©≥£ќ¬ѕ¬£ђ0.1mol/LNaHX»№“Ї÷–іж‘Џµƒјл„””–Na+°ҐX2-°ҐHX-°ҐH+°ҐOH-£ђіж‘ЏµƒЈ÷„”÷ї”–H2O£ђ«“c(H+)=0.01mol/L
ҐўЄ√»№“Ї÷–c(HX-)+c(X2-)=__________mol/L
ҐЏ≥£ќ¬ѕ¬£ђ0.1mol/LH2X»№“Ї÷–c(H+)________£®ћо>°Ґ<їт=£©0.11mol/L
£®3£©»фNaHX»№“Ї÷–Љм≤в≤їµљH2XЈ÷„”µЂњ…Љм≤вµљHX-£ђ‘т»№“Ї÷–c(H+)-c(OH-)________£®ћо>°Ґ<їт=£©c(X2-)£ђ»фHX-µƒµзјл∆љЇв≥£ эK=2.5°Ѕ10-6£ђ‘т0.4mol/LµƒNaHX»№“ЇµƒpH‘Љќ™__________
£®4£©≥£ќ¬ѕ¬ѕт20mL 0.05mol/Lƒ≥ѕ°ЋбH2X»№“Ї÷–µќ»л0.1mol/L∞±ЋЃ£ђ»№“Ї÷–”…ЋЃµзјл≥цјіµƒ«вјл„”≈®ґ»Ћжµќ»л∞±ЋЃћеїэ±дїѓ»зЌЉ£ђѕ¬Ѕ–Ј÷ќц’э»Јµƒ «________£®ћо„÷ƒЄіъЇ≈£©
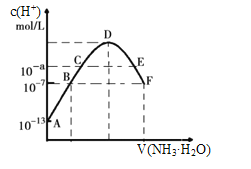
A.NaHX»№“Їњ…ƒ№ќ™Ћб–‘£ђ“≤њ…ƒ№ќ™Љо–‘
B.A°ҐB°ҐC»эµг»№“ЇµƒpH «÷рљ•Љх–°£ђD°ҐE°ҐF»эµг»№“ЇµƒpH «÷рљ•‘ціу
C.Eµг»№“Ї÷–јл„”≈®ґ»іу–°єЎѕµ£Їc(NH4+)>c(X2-)>c(OH-)>c(H+)
D.Fµг»№“Ї£Їc(NH4+)=2c(X2-)
£®5£©“—÷™ƒ≥їмЇѕ»№“Ї÷–Їђ”–4molNa2X°Ґ2molNa2CO3ЇЌ1molNaHCO3°£Ќщ»№“Ї÷–Ќ®»л4molCO2∆шће£ђ≥дЈ÷Јі”¶Їу∆шће»Ђ≤њ±їќь ’£ђЈі”¶ЇуNa2CO3ЇЌNaHCO3µƒќп÷ µƒЅњЈ÷±рќ™________£ђ________£®≤їњЉ¬«»хЋбЄщјл„”µƒµзјлЉ∞ЋЃљв£©
°Њір∞Є°њNaHX = H+ + X2- + Na+ > 0.1 0.1 < = 3 D 0mol 7mol
°Њљвќц°њ
£®1£©»ф≤вµ√іЋ»№“ЇµƒpH=1£ђ‘т—ќNaHX»№”ЏЋЃ ±µзјл≥цƒ∆јл„”°ҐЋбЄщјл„”ЇЌ«вјл„”£ї»фNaHX»№“Ї÷–ƒ№Љм≤вµљH2XЈ÷„”,‘тЋµ√чHX-њ…“‘ЋЃљв£ї
£®2£©»№“Ї≤їіж‘ЏH2X£ђЄщЊЁќпЅѕ ЎЇгЈ÷ќц£ї”…”Џ»№“Ї≤їіж‘ЏH2X£ђЋµ√чH2XµƒµЏ“ї≤љµзјл «Ќк»Ђµзјл£ђµЏґю≤љ≤њЈ÷µзјл£ђµЏ“ї≤љµзјл÷–H+ґ‘µЏґю≤љµƒµзјл≤ъ…ъЅЋ“÷÷∆£ї
£®3£©NaHX»№“Ї÷–Љм≤в≤їµљH2XЈ÷„”£ђµЂƒ№Љм≤вµљHX-£ђЋµ√чHX-÷їµзјл≤їЋЃљвґш є»№“Ї≥ Ћб–‘£ђ”…µзЇ… ЎЇгЉ∆Ћг£ђ‘ў”…µзјл≥£ эЉ∆Ћг«вјл„”≈®ґ»£ї
£®4£©”…AµгЋЃµзјл≥ц«вјл„”≈®ґ»ќ™10-13mol/Lњ…÷™Aµг»№“Ї÷–«в—хЄщјл„”≈®ґ»ќ™10-13mol/L£ђљбЇѕc£®H+£©=![]() Љ∆ЋгЋб»№“Ї÷–c£®H+£©£їЋж„≈∞±ЋЃµƒЉ”»л£ђ»№“ЇµƒЋб–‘Љх»х°ҐЉо–‘÷рљ•‘ц«њ£ђ»№“ЇµƒpH‘ціу£їE»№“Їѕ‘ ЊЋб–‘£ђЄщЊЁ—ќµƒЋЃљв‘≠јнјі±»љѕјл„”≈®ґ»£їЄщЊЁµзЇ… ЎЇгљбЇѕ»№“Ї≥ ÷––‘Ј÷ќцљвір£ї
Љ∆ЋгЋб»№“Ї÷–c£®H+£©£їЋж„≈∞±ЋЃµƒЉ”»л£ђ»№“ЇµƒЋб–‘Љх»х°ҐЉо–‘÷рљ•‘ц«њ£ђ»№“ЇµƒpH‘ціу£їE»№“Їѕ‘ ЊЋб–‘£ђЄщЊЁ—ќµƒЋЃљв‘≠јнјі±»љѕјл„”≈®ґ»£їЄщЊЁµзЇ… ЎЇгљбЇѕ»№“Ї≥ ÷––‘Ј÷ќцљвір£ї
£®5£©ƒ≥їмЇѕ»№“Ї÷–Їђ”–4mol Na2X°Ґ2mol Na2CO3ЇЌ1mol NaHCO3£ђNa2X°ҐNaHCO3ƒ№є≤іж£ђЋµ√чHX-µƒЋб–‘«њ”ЏћЉЋб«вЄщјл„”£ђЌщ»№“Ї÷–Ќ®»л4mol CO2∆шће£ђ≥дЈ÷Јі”¶Їу£ђ∆шће»Ђ≤њ±їќь ’£ђЋµ√чHX-µƒЋб–‘»х”ЏћЉЋб£ђЉі»№“Ї÷–≥эЅЋЈҐ…ъNa2CO3+CO2+H2O=2NaHCO3£ђїєЈҐ…ъЈі”¶Na2X+CO2+H2O=NaHX+NaHCO3£ђЄщЊЁЈі”¶Јљ≥ћ љљш––Љ∆Ћг°£
£®1£©Ґў»ф≤вµ√іЋ»№“ЇµƒpH=1£ђЋµ√чЄ√—ќ»№”ЏЋЃЌк»Ђµзјл£ђ…ъ≥…ƒ∆јл„”°ҐЋбЄщјл„”ЇЌ«вјл„”£ђЋщ“‘∆дµзјлЈљ≥ћ љќ™£ЇNaHX=Na++H++X2-£ђє ір∞Єќ™£ЇNaHX=Na++H++X2-£ї
ҐЏ»фNaHX»№“Ї÷–ƒ№Љм≤вµљH2XЈ÷„”£ђЋµ√ч «H2X «»хЋб£ђHX-њ…“‘≤њЈ÷ЋЃљв…ъ≥…H2X£ђґшNaHXЌк»Ђµзјл…ъ≥…Na+°ҐH+°ҐX2-£ђЋщ“‘c(X2-) >c(H2X)£їЄщЊЁќпЅѕ ЎЇг£ђc(X2-)+c(HX-)+c(H2X)=0.1mol/L£їє ір∞Єќ™£ЇNaHX = H+ + X2- + Na+£ї >£ї
£®2£©Ґў»№“Ї≤їіж‘ЏH2X£ђ”…ќпЅѕ ЎЇгњ…÷™£ђ0.1molL-1NaXB»№“Ї÷–c£®HX-£©+c£®X2-£©=0.1mol/L£їє ір∞Єќ™£Ї0.1£ї
ҐЏ”…”Џ»№“Ї÷–÷їіж‘ЏµƒЈ÷„”ќ™H2O£ђЋµ√чH2Xќ™÷–«њЋб£ђµзјлЈљ≥ћ љќ™H2X=H++HX-©pHX-H++X2-£ї”…ќпЅѕ ЎЇгњ…÷™c£®HX-£©+c£®X2-£©=c£®Na+£©=0.1mol/L£ї0.1molL-1NaHX»№“Їc£®H+£©=0.01molL-1£ђ‘т”–HX-µƒµзјлґ» «10%£ђH2XµƒµЏ“ї≤љµзјлќ™Ќк»Ђµзјл£ђµЏґю≤љќ™≤њЈ÷µзјл£ђ«“µЏ“ї≤љµзјл÷–H+ґ‘µЏґю≤љµƒµзјл≤ъ…ъЅЋ“÷÷∆£ђє H2X»№“Їc£®H+£©£Љ0.11molL-1£їє ір∞Єќ™£Ї£Љ£ї
£®3£©NaHX»№“Ї÷–Љм≤в≤їµљH2XЈ÷„”£ђµЂƒ№Љм≤вµљHX-£ђЋµ√чHX-÷їµзјл≤їЋЃљв£ђ»№“Ї÷–µзЇ… ЎЇгµ» љќ™£Їc(H+)+c(Na+)=c(OH-)+ c(HX-)+2c(X2-)£ђќпЅѕ ЎЇгµ» љќ™£Їc(Na+)= c(HX-)+c(X2-)£ђЅљ’яЇѕ≤Ґµ√µљµ» љ£Їc(H+)= c(OH-)+c(X2-)£ђ‘тc(H+)-c(OH-) =c(X2-)£їHX-µƒµзјл∆љЇв≥£ эK=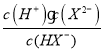 =
=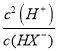
![]()
![]() =2.5°Ѕ10-6£ђЉ∆Ћг≥цc(H+)
=2.5°Ѕ10-6£ђЉ∆Ћг≥цc(H+)![]() 10-3 mol/L£ђpH‘Љќ™3£ђє ір∞Єќ™£Ї=£ї3£ї
10-3 mol/L£ђpH‘Љќ™3£ђє ір∞Єќ™£Ї=£ї3£ї
£®4£©A°ҐЋб»№“Ї÷–ЋЃµзјл≥ц«вјл„”≈®ґ»c£®H+£©µ»”Џ»№“Ї÷–«в—хЄщјл„”≈®ґ»c£®OH-£©£ђЉіc£®OH-£©®T10-13mol/L£ђ‘тЋб»№“Ї÷–c£®H+£©=![]() =
=![]() mol/L=0.1mol=2°Ѕ0.05molL-1£ђЋщ“‘ЋбH2Xќ™ґю‘™«њЋб£ђЉіNaHX»№“Їѕ‘Ћб–‘£ђє Aінќу£ї
mol/L=0.1mol=2°Ѕ0.05molL-1£ђЋщ“‘ЋбH2Xќ™ґю‘™«њЋб£ђЉіNaHX»№“Їѕ‘Ћб–‘£ђє Aінќу£ї
B°ҐЋж„≈∞±ЋЃµƒЉ”»л£ђ»№“ЇµƒЋб–‘Љх»х£ђ»№“ЇµƒpH‘ціу£ђЋщ“‘A°ҐB°ҐC»эµг»№“ЇµƒpH «÷рљ•‘ціуµƒ£ђDµг«°Ї√Јі”¶Ќк»Ђ£ђЋж„≈∞±ЋЃµƒЉ”»л£ђ»№“ЇµƒЉо–‘÷рљ•‘ц«њ£ђD°ҐE°ҐF»эµг»№“ЇµƒpH «÷рљ•‘ціу£ђє Bінќу£ї
C°ҐE»№“Їѕ‘ ЊЋб–‘£ђЅтЋбпІЇЌ∞±ЋЃµƒїмЇѕќп£ђµ√µљµƒ»№“Ї÷–пІЄщјл„”µƒЋЃљв≥ћґ»љѕ«њ£ђЋщ“‘c£®NH4+£©£Њc£®X2-£©£Њc£®H+£©£Њc£®OH-£©£ђє Cінќу£ї
D°ҐЄщЊЁµзЇ… ЎЇг£Їc£®H+£©+c£®NH4+£©®T2c£®X2-£©+c£®OH-£©£ђґш»№“Ї≥ ÷––‘c£®OH-£©®Tc£®H+£©£ђЋщ“‘c£®NH4+£©®T2c£®X2-£©£ђє D’э»Ј°£
ір∞Є—°D°£
£®5£©ƒ≥їмЇѕ»№“Ї÷–Їђ”–4mol Na2X°Ґ2mol Na2CO3ЇЌ1mol NaHCO3£ђNa2X°ҐNaHCO3ƒ№є≤іж£ђЋµ√чHX-µƒЋб–‘«њ”ЏћЉЋб«вЄщјл„”£ђЌщ»№“Ї÷–Ќ®»л4mol CO2∆шће£ђ≥дЈ÷Јі”¶Їу£ђ∆шће»Ђ≤њ±їќь ’£ђЋµ√чHX-µƒЋб–‘»х”ЏћЉЋб£ђЄщЊЁЈі”¶Na2CO3+CO2+H2O=2NaHCO3£ђњ…÷™2mol Na2CO3ƒ№…ъ≥…4molNaHCO3Ќђ ±ѕыЇƒґю—хћЉ2molCO2£ђїє”–2molCO2ЈҐ…ъЈі”¶Na2X+CO2+H2O=NaHX+NaHCO3£ђ…ъ≥…2molNaHCO3£ђЋщ“‘»№“Ї÷–√ї”–Na2CO3£ђNaHCO3ќ™7 mol£ђє ір∞Єќ™£Ї0mol£ї7mol°£

 ±Є’љ÷–њЉЇЃЉўѕµЅ–ір∞Є
±Є’љ÷–њЉЇЃЉўѕµЅ–ір∞Є