��Ŀ����
����Ŀ��Na2O2����Ҫ�Ļ���ԭ�ϣ����ж�����;��
��1��Na2O2���������ԣ����Խ�SO2����Ϊ�����ƣ�д���÷�Ӧ�Ļ�ѧ����ʽ��______________________���÷�Ӧ�У�Na2O2������Ϊ____________��������ԭ���������������������������������ǻ�ԭ��������
��2��Na2O2��CO2��Ӧ���Բ���������ijͬѧͨ������װ����֤Na2O2�ܷ���CO2��Ӧ�� (ͼ������̨��װ������ȥ)��

��װ��A��������_________��A�еĹ���Ϊ_______________��װ��B���Լ�������Ϊ__________________________
����Na2O2����CO2����װ��C�е�������_____________________________
��3����ʯ���Ǹ��������a���ռ����壬��ⷢ�ָ������м�������CO2���壨������������������˵������������CO2���岻��Ӧ����ͬѧ����������ף�Ȼ��װ��B�������������������Լ��������Ӻ�װ�ú��ٴν���ʵ�飬�����ռ������⣬���ֵõ������弸��������������ʵ����˵������������CO2���巴Ӧ��Ҫ_______________��
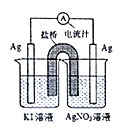
��4����һ������Na2O2����Ͷ�뵽�����������ӵ���Һ�У�NO3����HCO3-��CO32����Na������Ӧ��Ϻ���Һ������������Ŀ����������У���������Һ����ı仯��__________�������ӷ��ţ���
���𰸡�Na2O2��SO2=Na2SO4 ������ ��ƿ ̼���� ���������̼���� ����ɫ��ɰ�ɫ���� ��ˮ�IJ��� NO3��
��������
��1��Na2O2���������ԣ����Խ�SO2����Ϊ�����ƣ��ݴ�д����Ӧ�Ļ�ѧ����ʽ��
��2���ٸ�������A�Ľṹ���з���������������̼�����л�������ˮ�������������Ũ������и��
��Na2O2Ϊ��ɫ���壬��CO2������Ӧ���ɰ�ɫ����̼���ƣ�
��3�����������Ϣ���з�����
��4��Na2O2��ˮ��Ӧ�����������ƺ���������Һ�з���NaHCO3+NaOH= Na2CO3+H2O��Ӧ���ݴ˷�����Һ��n��HCO3-����n��CO32������n(Na+)��n��NO3�����仯���ɡ�
��1��Na2O2���������ԣ����Խ�SO2����Ϊ�����ƣ��÷�Ӧ�Ļ�ѧ����ʽΪ��Na2O2��SO2=Na2SO4���÷�Ӧ�У�Na2O2��-1�۵������͵�SO42-�е�-2�ۣ�Na2O2������ԭ��Ӧ������������
�ʴ��ǣ�Na2O2��SO2=Na2SO4 ����������
��2����װ��A����������ƿ��������̼���ƹ��巴Ӧ����������̼�����л�������ˮ�������������Ũ������и������װ��B���Լ�������Ϊ���������̼���壻
�ʴ��ǣ���ƿ��̼���ƣ����������̼���壻
��Na2O2Ϊ��ɫ���壬��CO2������Ӧ���ɰ�ɫ����̼���ƣ���װ��C�е������ǵ���ɫ��ɰ�ɫ���壻
�ʴ��ǣ�����ɫ��ɰ�ɫ���壻
��3��������Ϣ��֪������������CO2���岻��Ӧ��Ȼ��װ��B��������Ũ��������ˮ�����������������������Լ��������Ӻ�װ�ú��ٴν���ʵ�飬�����ռ������⣬���ֵõ������弸��������������ʵ����˵������������CO2���巴Ӧ��Ҫ��ˮ�IJ��룻
�ʴ��ǣ���ˮ�IJ��� ��
��4��Na2O2��ˮ��Ӧ�����������ƺ���������Ϊ����HCO3-+OH-= CO32��+H2O��Ӧ��������Һ��n��HCO3-����С��n��CO32��������n(Na+)����n��NO3�������䣻
�ʴ�ѡNO3����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�