��Ŀ����
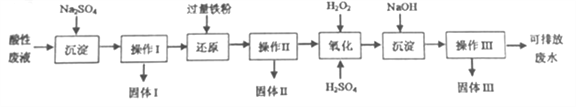
����Ŀ����ѧʵ���Ҳ����ķ�Һ�к��д�������Ⱦ���������ʣ�Ϊ�˱�����������ѧʵ���Ҳ����ķ�Һ���뾭����������ŷš�ij��ѧʵ���Ҳ��������Է�Һ�к���Fe3+��Cu2+��Ba2+���ֽ��������Ӻ�Cl-һ�������ӣ�ʵ������������������Է�Һ���д������Ի��ս������ⶨ����������������
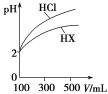
��֪ʵ���д��������Է�Һ�����Ϊ1L����pH�Ʋⶨ������H+���ʵ���Ũ��Ϊ0.10mol/L��
��ش��������⣺
(1)ʵ���в���I��II��III�ķ�����ͬ��������������Ϊ���ˡ�_________�����
(2)������������������Һ��ɫΪ�ػ�ɫ���÷�Ӧ�����ӷ���ʽΪ��__________��
(3)ʵ���г�������I ������Ϊ4.66g������II ������Ϊ15.2g����������ϡ�����ܽ����II ������˱�״���µ���ɫ����4.48L�������II �н���ͭ������Ϊ________g��
(4)ʵ���н�����III���г�����գ�ʹ���������˾ƾ��ơ����żܡ������ǡ��������⣬����_____�������ƣ������õ��ĺ���ɫ��������Ϊ40.0g����ԭ��Һ�������ӵ����ʵ���Ũ��Ϊ________��д��������̣���
���𰸡�ϴ��2Fe2++H2O2+2H+= 2Fe3++2H2O9.6����1.04mol/L
���������ں���Fe3+��Cu2+��Ba2+��Cl-�����Է�Һ�еμӹ���Na2SO4��Һ��ͨ������I�Ĺ��ˡ�ϴ�Ӳ�����ɵ�BaSO4��������Һ�м������Fe�ۿɻ�ԭ��Һ�е�Cu2+��ͨ���������Ĺ��ˡ�ϴ�Ӳ�����ɵ�Fe��Cu�Ļ�����������Һ�� �μӹ�����H2O2��Һ���ɽ���Һ�е�Fe2+����ΪFe3+���μӹ���NaOH��Һ�ɵ�Fe(OH)3���������������Ĺ��ˡ�ϴ�Ӳ�����ɵ�Fe(OH)3��������Һ���ŷţ�
(1)�з�����֪ʵ���в���I��II��III�ķ�����ͬ��������������Ϊ���ˡ�ϴ�ӡ����
(2)H2O2����Һ�е�Fe2+����ΪFe3+��������Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+= 2Fe3++2H2O��
(3)����I BaSO4����������Ϊ4.66g�����ʵ���Ϊ0.02mol������II Fe��Cu�Ļ���������Ϊ15.2g����������ϡ�����ܽ����II �������״���µ���ɫ����NOΪ4.48L�������ʵ���Ϊ0.2mol����Cu�����ʵ���Ϊxmol��Fe�����ʵ���Ϊymol����64x+56y=15.2��2x+3y=0.2��3�������غ�ʽ������ã�x=0.15��y=0.1��Cu������Ϊ64g/mol��0.15mol=9.6g��
(4)����������Ҫ�������н��У���ʵ���н�����III���г�����գ�ʹ���������˾ƾ��ơ����żܡ������ǡ��������⣬�������������õ��ĺ���ɫ����Fe2O3������Ϊ40.0g�������ʵ���Ϊ40.0g��160g/mol=0.25mol������Һ3�к��е�Fe2+Ϊ0.5mol��ԭ������Һ�к���H+�����ʵ���Ϊ0.10mol/L��1L=0.1mol�����ܽ�Fe���ɵ�Fe2+Ϊ0.1mol��![]() =0.05mol��ԭ������Һ�е�Cu2+�����ʵ���Ϊ0.15mol�����ܽ�Fe���ɵ�Fe2+Ϊ0.15mol������2Fe3++Fe=3Fe2+��ԭ��Һ�е�Fe3+�����ʵ���Ϊ(0.5mol-0.05mol-0.15mol)
=0.05mol��ԭ������Һ�е�Cu2+�����ʵ���Ϊ0.15mol�����ܽ�Fe���ɵ�Fe2+Ϊ0.15mol������2Fe3++Fe=3Fe2+��ԭ��Һ�е�Fe3+�����ʵ���Ϊ(0.5mol-0.05mol-0.15mol)![]() =0.2mol������ԭ������Һ�к���Ba2+��0.02mol��������Һ�ǵ����ԵĿ�֪n(Cl-)=n(Ba2+)��2+n(H+)+n(Fe3+)��3+n(Cu2+)��2=0.02mol��2+0.1mol+0.2mol��3+0.15mol��2=1.04mol����ԭ��Һ�������ӵ����ʵ���Ũ��Ϊ
=0.2mol������ԭ������Һ�к���Ba2+��0.02mol��������Һ�ǵ����ԵĿ�֪n(Cl-)=n(Ba2+)��2+n(H+)+n(Fe3+)��3+n(Cu2+)��2=0.02mol��2+0.1mol+0.2mol��3+0.15mol��2=1.04mol����ԭ��Һ�������ӵ����ʵ���Ũ��Ϊ![]() =1.04mol/L��
=1.04mol/L��

 ��У����ϵ�д�
��У����ϵ�д�