��Ŀ����
��1����4molSO2��2molO2����2L���ܱ������У���һ�������·�����Ӧ����10s��ﵽƽ�⣬���SO3��Ũ��Ϊ0.6mol?L-1����ش��������⣺
����O2��ʾ�ķ�Ӧ��ƽ������Ϊ______��
��ƽ��ʱSO2��ת����______��
��ƽ��ʱSO3���������Ϊ______��
��10sʱO2��Ũ��Ϊ______��
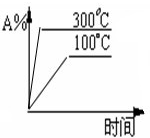
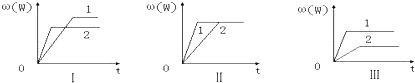
��2����֪ij���淴ӦmA��g��+nB��g��?qC��g�����ܱ������н��У���ͼ��ʾ��Ӧ�ڲ�ͬʱ��t���¶�T��ѹǿp�뷴Ӧ��B����������Ĺ�ϵ���ߣ�����ͼ�����
�ٻ�ѧ�������Ĺ�ϵ��m+n______q�������������������=����
�ڸ÷�Ӧ������ӦΪ______��Ӧ��������ȡ����ȡ���

����O2��ʾ�ķ�Ӧ��ƽ������Ϊ______��
��ƽ��ʱSO2��ת����______��
��ƽ��ʱSO3���������Ϊ______��
��10sʱO2��Ũ��Ϊ______��
��2����֪ij���淴ӦmA��g��+nB��g��?qC��g�����ܱ������н��У���ͼ��ʾ��Ӧ�ڲ�ͬʱ��t���¶�T��ѹǿp�뷴Ӧ��B����������Ĺ�ϵ���ߣ�����ͼ�����
�ٻ�ѧ�������Ĺ�ϵ��m+n______q�������������������=����
�ڸ÷�Ӧ������ӦΪ______��Ӧ��������ȡ����ȡ���

��1����һ���¶��£���4mol SO2��2molO2����2L���ܱ������У�c��SO2��=2mol/L��c��O2��=1mol/L����10s��ﵽƽ�⣬���SO3��Ũ��Ϊ0.6mol?L-1��
��ת�������������ʵ���Ũ��Ϊx����
2SO2 +O2 ?2SO3
��ʼ��mol?L-1�� 2 1 0
ת����mol?L-1�� 2x x 2x
ƽ�⣨mol?L-1�� 2-2x 1-x 2x
ƽ��ʱ���SO3��Ũ��Ϊ0.6mol?L-1��
����2x=0.6mol?L-1�����x=0.3mol?L-1��
��10s��������Ũ�ȱ仯Ϊ��c��O2��=0.3mol?L-1������v��O2��=
=
=0.03mol/��L?s����
�ʴ�Ϊ��0.03mol/��L?s����
��ƽ��ʱSO2��ת����Ϊ��
=
��100%=30%��
�ʴ�Ϊ��30%��
��ƽ��ʱ�������ڵ���Ũ��Ϊ��2-2x+1-x+2x=2.7mol?L-1������ƽ��ʱSO3���������
��100%=22.2%��
�ʴ�Ϊ��22.2%��
��ƽ��ʱ������Ũ�ȣ�1-x=0.7mol?L-1��
�ʴ�Ϊ��0.7mol?L-1��
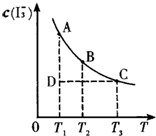
��2���ٶ��¶���ͬ���Ƚ�ѹǿ��ͬʱ�����Ƚ�����T1��p1������T1��p2�������ȳ��ֹյ㣬�ȵ���ƽ�⣬�ȳ��ֹյ�����߱�ʾ��ѹǿ�ߣ�����p1��p2��
��ͼ֪ѹǿԽ��B�ĺ���Խ�ߣ�����ƽ�����淴Ӧ���У�����ѹǿ��ƽ���������С�ķ����ƶ�������m+n��q��
�ʴ�Ϊ������
�ڶ�ѹǿ��ͬ���Ƚ��¶Ȳ�ͬʱ�����Ƚ�����T1��p2������T2��p2�������ȳ��ֹյ㣬�ȵ���ƽ�⣬�ȳ��ֹյ�����߱�ʾ���¶ȸߣ�����T1��T2����ͼ֪�¶�Խ�ߣ�B�ĺ���Խ�ͣ�����ƽ��������Ӧ���У������¶ȣ�ƽ�������ȷ����ƶ���������ӦΪ���ȷ�Ӧ��
�ʴ�Ϊ�����ȣ�
��ת�������������ʵ���Ũ��Ϊx����
2SO2 +O2 ?2SO3
��ʼ��mol?L-1�� 2 1 0
ת����mol?L-1�� 2x x 2x
ƽ�⣨mol?L-1�� 2-2x 1-x 2x
ƽ��ʱ���SO3��Ũ��Ϊ0.6mol?L-1��
����2x=0.6mol?L-1�����x=0.3mol?L-1��
��10s��������Ũ�ȱ仯Ϊ��c��O2��=0.3mol?L-1������v��O2��=
| ��c |
| t |
| 0.3mol?L-1 |
| 10s |
�ʴ�Ϊ��0.03mol/��L?s����
��ƽ��ʱSO2��ת����Ϊ��
| n(��Ӧ) |
| n(����) |
| 0.6 |
| 2 |
�ʴ�Ϊ��30%��
��ƽ��ʱ�������ڵ���Ũ��Ϊ��2-2x+1-x+2x=2.7mol?L-1������ƽ��ʱSO3���������
| 0.6 |
| 2.7 |
�ʴ�Ϊ��22.2%��
��ƽ��ʱ������Ũ�ȣ�1-x=0.7mol?L-1��
�ʴ�Ϊ��0.7mol?L-1��
��2���ٶ��¶���ͬ���Ƚ�ѹǿ��ͬʱ�����Ƚ�����T1��p1������T1��p2�������ȳ��ֹյ㣬�ȵ���ƽ�⣬�ȳ��ֹյ�����߱�ʾ��ѹǿ�ߣ�����p1��p2��
��ͼ֪ѹǿԽ��B�ĺ���Խ�ߣ�����ƽ�����淴Ӧ���У�����ѹǿ��ƽ���������С�ķ����ƶ�������m+n��q��
�ʴ�Ϊ������
�ڶ�ѹǿ��ͬ���Ƚ��¶Ȳ�ͬʱ�����Ƚ�����T1��p2������T2��p2�������ȳ��ֹյ㣬�ȵ���ƽ�⣬�ȳ��ֹյ�����߱�ʾ���¶ȸߣ�����T1��T2����ͼ֪�¶�Խ�ߣ�B�ĺ���Խ�ͣ�����ƽ��������Ӧ���У������¶ȣ�ƽ�������ȷ����ƶ���������ӦΪ���ȷ�Ӧ��
�ʴ�Ϊ�����ȣ�

��ϰ��ϵ�д�
 ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
�����Ŀ