��Ŀ����
����Ŀ��������������SRB(�����λ�ԭ��)������ˮ�е��л��SO42-���ؽ�����Ⱦȡ�����µĽ�չ��
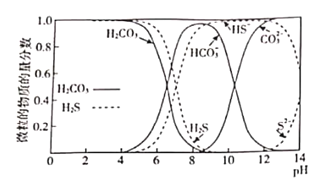
��1����ͼ��ʾH2CO3��H2S��ˮ��Һ�и��������ʵ���������pH�ı仯���ߡ�ij����ˮ��pH=8.5����SRB�������£���ˮ�е��л���(��ҪΪCH3COO��)��SO42-��ԭΪ-2����Ļ�����������ӷ���ʽ��ʾ�ù����еĻ�ѧ�仯��_______________��
��2��SRB��ȥ��ˮ���л����ͬʱ�����ɵ�H2S�������ڹ��������أ�ijpH�¸�����ȼ�ϵ�صĹ���ԭ������ͼ��ʾ��

��д����������ĵ缫��Ӧ��________________��
�ڸ����ҵ�pH�淴Ӧ���з����ı仯��__________(����С��������)����ϵ缫��Ӧ����pH�仯��ԭ��__________��
��3��SRB�����ڴ�����ˮ�к��ؽ�����(Sb)�����ӡ�
��ͨ��������Ӧ��Sb(OH)6��ת��ΪSb2S3��ȥ��ת���������е������ɡ���ɵ�һ����Ӧ�����ӷ���ʽ��
��һ���� Sb(OH)6��+ H2S ===1 + 1 ��+ H2O��_______________________
�ڶ�����3H2S +2SbO2��+2H+ ===Sb2S3�� +4H2O
��ijС��ģ��ʵ��ʱ����amL��SbԪ��b mg��L-1 �ķ�ˮ�����ȡ________gKSb(OH)6(��Է�������Ϊ263)����ʾ���г�����ʽ���ɡ�
���𰸡�SO42- + CH3COO- = HS-+2HCO3- O2 + 4H+ + 4e-= 2H2O ���� �����缫��Ӧ����ʽH2S + 4H2O - 8e-=SO42- + 10H+����������Ӧ����1 mol SO42-ʱ������10 mol H+��ͬʱ��8mol H+ͨ�����ӽ���Ĥ���������ң�����pH��С 1Sb(OH)6�� +1H2S =1SbO2��+1S��+4H2O ![]()
��������
(1)����ͼʾ����pH=8.5��SRB�������£���ˮ�е��л���(��ҪΪCH3COO-)��SO42-��ӦΪHS-��HCO3-��
(2)��ͨ�������ĵ缫Ϊԭ��ص���������������Һ�������õ����ӷ�Ӧ����ˮ��
�ڸ���ͼʾ������ʧ���ӷ���������Ӧ�������ᣬ���������Ӧ�����жϣ�
(3)�ٽ�ϵڶ����ķ���ʽ��֪����Ӧ��Sb(OH) 6-ת��ΪSbO2-��SbԪ�ػ��ϼ�+6�۽���Ϊ+3�ۣ���H2S����Ԫ�ػ��ϼ�-2������Ϊ0�ۣ����ݵ�ʧ�����غ��ԭ���غ���ƽ�õ����ӷ���ʽ��
�ڸ���SbԪ���غ����KSb(OH)6��������
(1)ij����ˮ��pH=8.5����SRB�������£���ˮ�е��л���(��ҪΪCH3COO-)��SO42-��ԭΪ-2����Ļ��������ͼ�б仯���ߺ�pH���������ɵ�������Ҫ��HS-��HCO3-����Ӧ�����ӷ���ʽ��SO42-+CH3COO-![]() HS-+HCO3-���ʴ�Ϊ��SO42-+CH3COO-
HS-+HCO3-���ʴ�Ϊ��SO42-+CH3COO-![]() HS-+HCO3-��
HS-+HCO3-��
(2)��ͨ�������ĵ缫Ϊԭ��ص�������ԭ�������������ԭ��Ӧ����������Һ�������õ����ӷ�Ӧ����ˮ���缫��ӦΪO2+4H++4e-=2H2O���ʴ�Ϊ��O2+4H++4e-=2H2O��
��SRB��ȥ��ˮ���л����ͬʱ�����ɵ�H2S�������ڹ��������أ�ͨ��H2S��Ϊԭ��صĸ���������������ʧ���ӷ���������Ӧ�������ᣬ��Һ��pH��С�������ĵ缫��Ӧ��H2S+4H2O-8e-=SO42-+10H+��ÿ����1mol�������������10mol�����ӣ���������ĵ缫��Ӧ��O2+4H++4e-=2H2O��֪����8mol������ͨ������Ĥ����������������pH��С���ʴ�Ϊ����С�������缫��Ӧ��H2S+4H2O-8e-=SO42-+10H+��ÿ����1mol�������������10mol�����ӣ�ͬʱ��8mol������ͨ������Ĥ����������������pH��С��
(3)�ٽ�Sb(OH)6��ת��ΪSb2S3��ȥ��ת���������е������ɣ���ϵڶ����ķ���ʽ��֪����Ӧ��Sb(OH) 6-ת��ΪSbO2-��SbԪ�ػ��ϼ�+6�۽���Ϊ+3�ۣ�����ת��3e-����H2S����Ԫ�ػ��ϼ�-2������Ϊ0�ۣ�����ת��2e-�����ݵ�ʧ�����غ��ԭ���غ���ƽ�õ����ӷ���ʽ��Sb(OH) 6-+H2S�TSbO2-+S��+4H2O���ʴ�Ϊ��1Sb(OH)6��+1H2S =1SbO2��+1S��+4H2O��
��ijС��ģ��ʵ��ʱ����a mL��SbԪ��b mgL-1 �ķ�ˮ����ˮ�к���SbԪ�ص����ʵ���=![]() ������SbԪ���غ㣬���ȡKSb(OH)6������=
������SbԪ���غ㣬���ȡKSb(OH)6������=![]() ��263g/mol=
��263g/mol=![]() g���ʴ�Ϊ��
g���ʴ�Ϊ��![]() ��
��

 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�����Ŀ����ϩ����Ҫ�Ļ���ԭ�ϡ���CO2���������ȡ��ϩ��2CO2(g)+6H2(g)![]() C2H4(g)+4H2O(g) ��H<0
C2H4(g)+4H2O(g) ��H<0

(1)���÷�Ӧ��ϵ�������淴Ӧ���̱仯��ϵ��ͼ��ʾ����÷�Ӧ����H=_________kJ��mol1��(�ú�a��b��ʽ�ӱ�ʾ)
(2)���ֻ�ѧ���ļ��������ʾ��ʵ����������Ӧ����H= 152 kJ��mol1������е�x=___________��
��ѧ�� | C=O | H-H | C=C | C-H | O-H |
����/(kJ��mol-1) | 803 | 436 | x | 414 | 463 |
(3)��1 L�����ܱ�������ͨ��1 mol CO2��n mol H2����һ�������·���������Ӧ�����CO2��ת������(CO2)�뷴Ӧ�¶ȡ�Ͷ�ϱ�X[ n(H2)/n(CO2 )]�Ĺ�ϵ��ͼ��ʾ��

��X1_________(����>������<������=������ͬ)X2��
��ƽ�ⳣ��KA_______KB��KB________KC��
����B��ʱX=3����ƽ�ⳣ��KB=_____________(���������г���ʽ����)��
�����д�ʩ��ͬʱ��������Ӧ���ʺ����CO2ת���ʵ�����__________��
a�������¶� b��������� c������Ͷ�ϱ�X d��������ӷ�Ӧ��ϵ�з������

(4)��ϡ����Ϊ�������Һ������̫���ܵ�ؽ�CO2ת��Ϊ��ϩ�Ĺ���ԭ����ͼ��ʾ����N���ϵĵ缫��ӦʽΪ��_______________���õ��������������ܷ�Ӧ�Ļ�ѧ����ʽΪ��__________________��
����Ŀ��ijʵ��С���ڳ����½��е�ⱥ��Ca(OH)2��Һ��ʵ�飬ʵ��װ����������±���
��� | I | II |
װ�� |
|
|
���� | �����������������ݣ�b����a���ࣻa����Һ������ɫ���ǣ��ð�ɫ���Ǽ������������ݲ��� | �����������������ݣ�d����c���ࣻc���������������ɫ���壻c����Һδ����ɫ���� |
���й���ʵ������Ľ��������ۣ���ȷ���ǣ� ��
A. a����Һ������ɫ���ǵ���Ҫԭ���ǵ���������ˮ������Ca(OH)2����
B. b���������ݣ�4OH�� ��4e�� === O2�� ��2H2O
C. c�������ڣ�Cu ��2e�� ��2OH�� === CuO ��H2O
D. d���缫��Ӧ�ķ�����������ˮ�ĵ���




