��Ŀ����
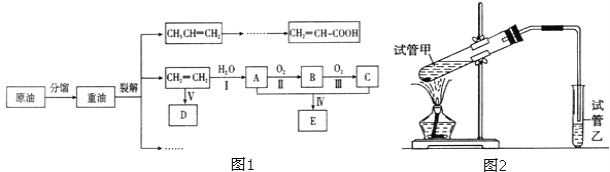
����Ŀ����ϩ������ʯ�͵���Ҫ�л�����ԭ�ϣ������ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ�����ͼ1��·�ش�
��֪��2CH3CHO+O2![]() 2CH3COOH
2CH3COOH
��1���������������������仯����______________������ţ����ٷ�����ѽ�
��2����ӦII�Ļ�ѧ����ʽ��______________��
��3��DΪ�߷��ӻ������������������ְ�װ���ϣ���ṹ��ʽ��______________��
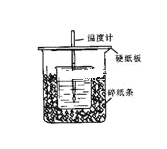
��4��E������ζ�����ʣ���ʵ�����п���ͼ2װ����ȡ��
�ٷ�ӦIV�Ļ�ѧ����ʽ��______________��
���Թ����еĵ�����Ҫ���ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ����______________��
��5��������ϩ������Ľṹ�����ʽ�����ȣ������л���CH2=CH-COOH��˵����ȷ����______________��
����CH3COOH��Ϊͬϵ��
�ڿ�����NaHCO3��Һ��Ӧ�ų�CO2����
����һ�������¿��Է����������ӳɡ�������Ӧ
���𰸡��� 2CH3CH2OH+O2![]() 2CH3CHO+2H2O
2CH3CHO+2H2O ![]() CH3COOH+CH3CH2OH
CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O ��ֹ���� �ڢ�
CH3COOCH2CH3+H2O ��ֹ���� �ڢ�
��������
ʯ�;������õ��е㲻ͬ�ĸ�����֣����õ��������ѽ��õ����в����ͼ��Ķ����������ϩ����ϩ�ȣ���ϩ����Ӧ����ˮ�����ӳɷ�Ӧ�����Ҵ����Ҵ�����Ӧ��������������������Ӧ������ȩ����ȩ����Ӧ������������������Ӧ�������ᣬ�Ҵ���������Ũ�����������������·�����Ӧ�����������������ݴ˷�����
(1)ʯ�ͷ���ʱ�����������ʣ��������������仯���ѽ�ʱ���������ʣ��������ڻ�ѧ�仯�����������������������仯���Ǣ٣�
(2)��ͭ���������������������£��Ҵ��ܱ���������������ȩ��ˮ����Ӧ����ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
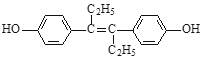
(3)��ϩ�����Ӿ۷�Ӧ���ɾ���ϩ����ṹ��ʽΪ![]() ��
��
(4)����ϩ��ˮ��Ӧ�����Ҵ����Ҵ��ڴ���������������ȩ����ȩ�����������������ᣬ��Ũ���������������������£��Ҵ������ᷢ��������Ӧ��������������ˮ����ӦIV�Ļ�ѧ����ʽΪ��CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
���Թ����еĵ�����Ҫ���ڱ���̼������Һ��Һ���ϣ���Ϊ�淴Ӧ�������Ҵ�����������Һ�е��ܽ�Ƚϴ��ܲ���Һ���¼�������������˵�������Һ���ϵ�Ŀ���Ƿ�ֹ������
(5)��CH2=CH-COOH��CH3COOH�ṹ�����ƣ����ڷ��������Ҳû�����һ��-CH2ԭ���ţ����Զ��߲�����ͬϵ�����CH2=CH-COOH�к����Ȼ����ܺ�̼�����Ʒ�Ӧ���ɶ�����̼����ȷ����CH2=CH-COOH����̼̼˫�����ܷ����ӳɷ�Ӧ��������Ӧ���Ӿ۷�Ӧ�ȣ����Ȼ��ܷ���������Ӧ����ȷ����ѡ�ڢۡ�