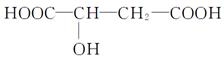��Ŀ����
���л��ﻯѧ������
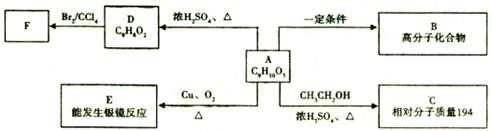
ƻ����㷺������ƻ����ˮ���Ĺ����У���һ�ֳ��õ�ʳƷ���Ӽ������ⶨ��ƻ�������Է�������Ϊ134��������Ԫ�ص���������Ϊ��w(c)="35.82%" W(H)=4.86%,����Ϊ�������д���5�ֲ�ͬ��ѧ������Hԭ�ӡ�1molƻ��������2molNaHCO3��ȫ��Ӧ������������Na��Ӧ����1.5molH2�ġ�����ϩΪԭ���˹��ϳ�ƻ�������·���£�
��֪��
��ش��������⣺
��1��ƻ����ķ���ʽΪ_______��A���ʵ�����Ϊ_______��
��2��F�к��еĹ�����������_______��G+B��H�ķ�Ӧ������_______��
��3���ںϳ���·�У�C��D��һ���跴Ӧ��Ŀ����_____��
��4��D��E��Ӧ�Ļ�ѧ����ʽΪ_________��
��5��ƻ�����NaHCO3��ȫ��Ӧ�Ļ�ѧ����ʽΪ________��
��6����ƻ���Ậ����ͬ����������Ĺ����ŵ�ͬ���칹��Ľṹ��ʽΪ____��
ƻ����㷺������ƻ����ˮ���Ĺ����У���һ�ֳ��õ�ʳƷ���Ӽ������ⶨ��ƻ�������Է�������Ϊ134��������Ԫ�ص���������Ϊ��w(c)="35.82%" W(H)=4.86%,����Ϊ�������д���5�ֲ�ͬ��ѧ������Hԭ�ӡ�1molƻ��������2molNaHCO3��ȫ��Ӧ������������Na��Ӧ����1.5molH2�ġ�����ϩΪԭ���˹��ϳ�ƻ�������·���£�


��֪��
��ش��������⣺
��1��ƻ����ķ���ʽΪ_______��A���ʵ�����Ϊ_______��
��2��F�к��еĹ�����������_______��G+B��H�ķ�Ӧ������_______��
��3���ںϳ���·�У�C��D��һ���跴Ӧ��Ŀ����_____��
��4��D��E��Ӧ�Ļ�ѧ����ʽΪ_________��
��5��ƻ�����NaHCO3��ȫ��Ӧ�Ļ�ѧ����ʽΪ________��
��6����ƻ���Ậ����ͬ����������Ĺ����ŵ�ͬ���칹��Ľṹ��ʽΪ____��
��1��C4H6O5 �Ҵ�
��2�� �ǻ����Ȼ� �ӳɷ�Ӧ��
��3����C����������Brԭ�ӣ�
��4��Br��CH2��COOH+2NaOH HO��CH2��COONa+NaBr+H2O
HO��CH2��COONa+NaBr+H2O
��5��

��6��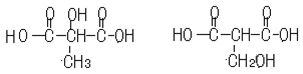
��2�� �ǻ����Ȼ� �ӳɷ�Ӧ��
��3����C����������Brԭ�ӣ�
��4��Br��CH2��COOH+2NaOH
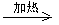 HO��CH2��COONa+NaBr+H2O
HO��CH2��COONa+NaBr+H2O��5��

��6��
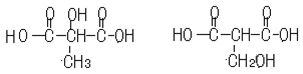
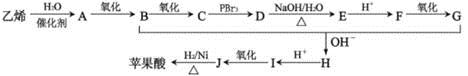
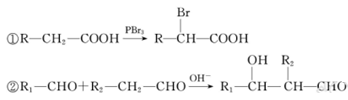
��1������̼���⡢��ԭ�ӵ�������������Է��������������ƻ����ķ���ʽ��C4H6O5����ϩ��ˮ�ڴ�������ʱ�����ӳɷ�Ӧ����A���Ҵ���������������̼ԭ�Ӳ����֪��B����ȩ��C����������Ϣ�ٽ�һ���ƶϣ�D��BrCH2COOH��E��HOCH2COONa��F��HOCH2COOH��G��OHC-CH2COOH��������Ϣ�ڣ����ɵ�HΪNaOOC-CH��OH��-CH2CHO���ữ���I��HOOCH��OH��-CH2CHO��I������ΪJ��J��HOOC-CO-CH2-COOH��������������õ�ƻ���HOOC-CH��OH��-CH2COOH��
��3���������ͼ֪��C��D��һ����Ӧ��Ŀ����Ϊ����C������Brԭ�ӡ�
��4��D��E�Ļ�ѧ����ʽ��Br��CH2��COOH+2NaOH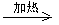 HO��CH2��COONa+NaBr+H2O
HO��CH2��COONa+NaBr+H2O
��5��ƻ�����NaHCO3��ȫ��Ӧ�Ļ�ѧ����ʽ��

��6����ƻ���Ậ����ͬ�������Ŀ�Ĺ����ŵĽṹΪ��

��3���������ͼ֪��C��D��һ����Ӧ��Ŀ����Ϊ����C������Brԭ�ӡ�
��4��D��E�Ļ�ѧ����ʽ��Br��CH2��COOH+2NaOH
 HO��CH2��COONa+NaBr+H2O
HO��CH2��COONa+NaBr+H2O��5��ƻ�����NaHCO3��ȫ��Ӧ�Ļ�ѧ����ʽ��

��6����ƻ���Ậ����ͬ�������Ŀ�Ĺ����ŵĽṹΪ��


��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
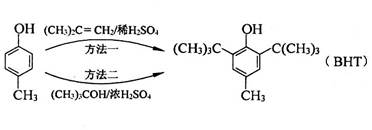
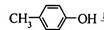 ��BHT��Ϊͬϵ��
��BHT��Ϊͬϵ��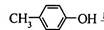 �ܷ���������Ӧ
�ܷ���������Ӧ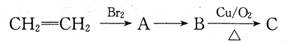

 ��ϵͳ����Ϊ �������ķ�Ӧ����Ϊ ��
��ϵͳ����Ϊ �������ķ�Ӧ����Ϊ ��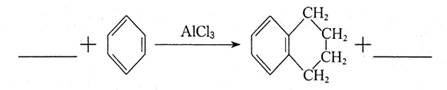
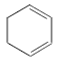 ��ʹ������Ȼ�̼��Һ�����Ը��������ɫ������Ӧԭ����ͬ
��ʹ������Ȼ�̼��Һ�����Ը��������ɫ������Ӧԭ����ͬ