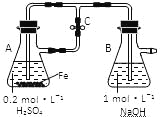
��Ŀ����
����Ŀ��������һ����Ҫ�Ĺ�ҵԭ�ϡ���ͼ��һ����ȡ����֤�������ֻ�ѧ���ʵ�ʵ��װ�á���֪��Cl2��2KI=I2��2KCl��

��1��д��ʵ������ȡ������Ӧ�Ļ�ѧ����ʽ___��
��2��װ��B��������___��
��3����������Cl2ͨ���۲쵽װ��C����Һ���___ɫ��
��4����Cl2����ͨ��ʱ��װ��D�и������ɫ�����ܷ���ɫ��Ϊʲô��__��
��5����Ҫ֤��Cl2��Ư���ԣ��������װ��D֮ǰ��һ��װ��__��ϴ��ƿ��
��6��װ��E��������__���÷�Ӧ�����ӷ���ʽΪ___��
���𰸡�MnO2+ 4HCl��Ũ��![]() MnCl2+Cl2��+2H2O ����HCl �� ����ɫ����ΪCl2�������H2O(g)��Ӧ����HClO��ʹ��ɫ������ɫ Ũ���� ��ȥ�����Cl2 Cl2��2OH��=Cl����ClO����H2O
MnCl2+Cl2��+2H2O ����HCl �� ����ɫ����ΪCl2�������H2O(g)��Ӧ����HClO��ʹ��ɫ������ɫ Ũ���� ��ȥ�����Cl2 Cl2��2OH��=Cl����ClO����H2O
��������
װ��AΪ��ȡ������װ�ã�װ��BΪ��HCl��װ�ã�CΪ��֤�������������ԣ�DΪ��֤���������û��Ư���ԣ�EΪβ������װ�á�
��1��ʵ����ͨ���ö���������Ũ���Ṳ����ȡ����������ʽΪMnO2+ 4HCl��Ũ��![]() MnCl2+Cl2��+2H2O��
MnCl2+Cl2��+2H2O��
��2��Ũ������лӷ��ԣ��ڼ���ʱ����HCl�������壬��ʳ��ˮ������HCl���壬�ҽ����������ܽ�ȣ�
��3������Cl2ͨ����������KI��Ӧ����I2��I2����������Һ����ɫ��
��4��Cl2����ͨ��װ��D�У���������������Һʱ������������ˮ������ʹ�������ɫ������ó�ʪ�����ɴ����ᣬ�Ӷ�ʹ��ɫ������ɫ��
��5����Ҫ֤������Cl2�Ƿ���Ư���ԣ�����������Ϊ����ģ�����C��D֮�����Ӹ���װ�ã���Ũ���
��6�������ж�������ֱ���ŷŵ������У���NaOH��ҺΪ����δ��Ӧ����������Ӧ�����Ȼ��ơ��������ƺ�ˮ�����ӷ�ӦΪCl2��2OH��=Cl����ClO����H2O��