��Ŀ����
��9�֣����к͵ζ����ⶨ�ռ�Ĵ��ȣ����ռ��в��������ᷴӦ�����ʣ��Ը���ʵ��ش�
��1��ȷ��ȡ�ռ���Ʒ4.1g������Ʒ���250mL�Ĵ���Һ����Ҫ��������С�ձ�����Ͳ���������� �� ��(������)
��2��ȡ10.00mL����Һ���� ��ȡע����ƿ�С�(������)
��3����0.2010mol/L��������Һ�ζ������ռ���Һ���ζ�ʱ���� ������ ������ע�� ��ֱ���ζ��յ㡣
��4���������вⶨ���ݣ������õ��������ݣ���������ռ���Һ��Ũ�ȣ� ��
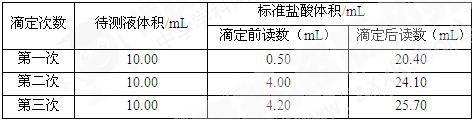
��5�����������ⶨ���ݣ������õ��������ݣ������ռ�Ĵ��� ��
��1��ȷ��ȡ�ռ���Ʒ4.1g������Ʒ���250mL�Ĵ���Һ����Ҫ��������С�ձ�����Ͳ���������� �� ��(������)
��2��ȡ10.00mL����Һ���� ��ȡע����ƿ�С�(������)
��3����0.2010mol/L��������Һ�ζ������ռ���Һ���ζ�ʱ���� ������ ������ע�� ��ֱ���ζ��յ㡣
��4���������вⶨ���ݣ������õ��������ݣ���������ռ���Һ��Ũ�ȣ� ��

��5�����������ⶨ���ݣ������õ��������ݣ������ռ�Ĵ��� ��
��1��250mL����ƿ����ͷ�ιܡ���ƽ��
��2�����ü�ʽ�ζ�������ȡ��Ҳ��ʹ����Һ������ȡ
��3���ζ������У��ζ�ʱ������ת��ʽ�ζ��ܵIJ������������ֲ�ͣ��ҡ����ƿ������ע����ƿ����Һ��ɫ�ı仯��ֱ���ζ��յ㡣
��4��V(NaOH)="10.00mL " V(HCl)=[(20.40mL-0.50mL)+(24.10mL-4.00)]/2=20.00mL
���ݣ�c(NaOH)V(NaOH)="c(HCl)V(HCl) " ��c(NaOH)=0.4020mol/L
��5����Ʒ��m(NaOH)=0.4020mo
 l/L��0.250L��40g/mol=4.02g
l/L��0.250L��40g/mol=4.02g���ռ�Ĵ���Ϊ��4.02g/4.1g��100%=98%
��

��ϰ��ϵ�д�
�����Ŀ
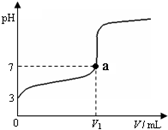
 Һ����ƿδ��NaOH��Һ��
Һ����ƿδ��NaOH��Һ�� ϴ
ϴ