��Ŀ����
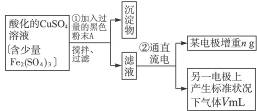
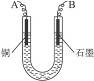
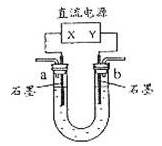
��֪��pHΪ4��5�Ļ����У�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⡣ijѧ�����õ�ⴿ��CuSO4��Һ�ķ����������ݵ缫������ͭ�������Լ��缫�ϲ��������������ⶨͭ�����ԭ����������ʵ���������£�
ͼ4-16
�Իش��������⣺
(1)������������A�Ļ�ѧʽΪ________________������A��������___________________,
(2)����������ò���������ͼ4-16��ʾ����AӦ��ֱ����Դ��__________����
(3)��ʼ��һ��ʱ�䣬��U�ι��ڿɹ۲쵽��������____________�������ܷ�Ӧ�����ӷ���ʽΪ________________________��
(4)����ʵ���������Ҫ����__________(����ĸ����ͬ)��������Ҫ����________________��
E.���п������ڵ�����£��缫��ɱ�����õ��º�ɷ�
(5)ͭ�����ԭ������Ϊ______________________��


ͼ4-16
�Իش��������⣺
(1)������������A�Ļ�ѧʽΪ________________������A��������___________________,
(2)����������ò���������ͼ4-16��ʾ����AӦ��ֱ����Դ��__________����
(3)��ʼ��һ��ʱ�䣬��U�ι��ڿɹ۲쵽��������____________�������ܷ�Ӧ�����ӷ���ʽΪ________________________��
(4)����ʵ���������Ҫ����__________(����ĸ����ͬ)��������Ҫ����________________��
| A���������ǰ�缫������ |
| B������缫�ں�ɡ�����ǰ������������ˮ��ϴ |
| C�����µ���缫�ϵ�ͭ������ϴ������ |
| D���缫�ں�ɳ����IJ������밴��ɡ��������ٺ�ɡ��ٳ����������� |
(5)ͭ�����ԭ������Ϊ______________________��
(1)CuO ����H+ʹ��Һ��pH���ߣ�Fe3+ˮ������Fe(OH)3���� (2)��
(3)��B������ɫ��ζ�������ɣ���A���к�ɫ�������ɣ��������Һ����ɫ��dz2Cu2++2H2O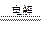 2Cu+4H++O2�� (4)ABDE C (5)
2Cu+4H++O2�� (4)ABDE C (5)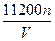
(3)��B������ɫ��ζ�������ɣ���A���к�ɫ�������ɣ��������Һ����ɫ��dz2Cu2++2H2O
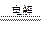 2Cu+4H++O2�� (4)ABDE C (5)
2Cu+4H++O2�� (4)ABDE C (5)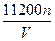
2Cu2++2H2O 2Cu��4H++O2����
2Cu��4H++O2����
n(Cu)��n(O2)=2��1��n(Cu)= mol,n(O2)=
mol,n(O2)=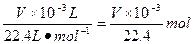
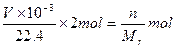
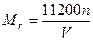
 2Cu��4H++O2����
2Cu��4H++O2����n(Cu)��n(O2)=2��1��n(Cu)=
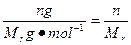 mol,n(O2)=
mol,n(O2)=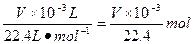
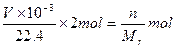
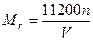

��ϰ��ϵ�д�
�����Ŀ
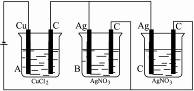
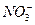 )
)
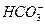 )��ʳ��ˮװ��U�ι��У�����ܿ���ijһ�缫��������ʳ��ˮ���ǵ�������ɻ��ǵ���������Ҫ�ǡ�( )
)��ʳ��ˮװ��U�ι��У�����ܿ���ijһ�缫��������ʳ��ˮ���ǵ�������ɻ��ǵ���������Ҫ�ǡ�( ) Zn
Zn