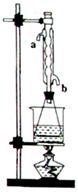
��Ŀ����
(10��)��ҵ�ϳ��÷���м����һ��Ũ�ȵ�������Һ�Ʊ��̷�( FeSO4��7H2O )��
��1������98% 1.84 g/cm3��Ũ��������������28%��������Һ����Ũ������ˮ�������ԼΪ1�� �� ��
��2��Ϊ�ⶨij�����ڿ������̷���Ʒ��Fe2+�������ʣ�ijͬѧ�������ʵ�飺ȡһ��������Ʒ����������ϡ�����У�Ȼ�����5.00 g���۳�ַ�Ӧ���ռ���224 mL����״�������壬ʣ���������Ϊ3.88 g����÷�Ӧ�����Һ��Fe2+�����ʵ���Ϊ0.14 mol������Fe3+���������Ʒ��Fe2+���ӵ�������Ϊ �� ��
��3����������泥�(NH4)2SO4��FeSO4��6H2O��(�׳�Ī����)�����̷��ȶ�����������ԭ�ζ������г���������Fe2+�ı���Һ����ȡ0.4 g Cu2S��CuS�Ļ������������Һ����40 mL 0.150 mol/L KMnO4��Һ������������Ӧ���£�
8MnO4����5Cu2S��44H����10Cu2����5SO2��8Mn2����22H2O
6MnO4����5CuS��28H����5Cu2����5SO2��6Mn2����14H2O
��Ӧ�������Һ���Ͼ�SO2��ʣ���KMnO4ǡ����V mL 0.2 mol/L (NH4)2Fe(SO4)2��Һ��ȫ��Ӧ����֪��MnO4����Fe2����H������Mn2����Fe3+��H2O��δ��ƽ��
��V��ȡֵ��ΧΪ �� ��
����V=35���Լ���������CuS������������
��1������98% 1.84 g/cm3��Ũ��������������28%��������Һ����Ũ������ˮ�������ԼΪ1�� �� ��
��2��Ϊ�ⶨij�����ڿ������̷���Ʒ��Fe2+�������ʣ�ijͬѧ�������ʵ�飺ȡһ��������Ʒ����������ϡ�����У�Ȼ�����5.00 g���۳�ַ�Ӧ���ռ���224 mL����״�������壬ʣ���������Ϊ3.88 g����÷�Ӧ�����Һ��Fe2+�����ʵ���Ϊ0.14 mol������Fe3+���������Ʒ��Fe2+���ӵ�������Ϊ �� ��
��3����������泥�(NH4)2SO4��FeSO4��6H2O��(�׳�Ī����)�����̷��ȶ�����������ԭ�ζ������г���������Fe2+�ı���Һ����ȡ0.4 g Cu2S��CuS�Ļ������������Һ����40 mL 0.150 mol/L KMnO4��Һ������������Ӧ���£�
8MnO4����5Cu2S��44H����10Cu2����5SO2��8Mn2����22H2O
6MnO4����5CuS��28H����5Cu2����5SO2��6Mn2����14H2O
��Ӧ�������Һ���Ͼ�SO2��ʣ���KMnO4ǡ����V mL 0.2 mol/L (NH4)2Fe(SO4)2��Һ��ȫ��Ӧ����֪��MnO4����Fe2����H������Mn2����Fe3+��H2O��δ��ƽ��
��V��ȡֵ��ΧΪ �� ��
����V=35���Լ���������CuS������������
��1��4.6��3�֣�
��2��16.7%��1/6����3�֣�
��3����25��V��50��2�֣� ��60%��2�֣�
��2��16.7%��1/6����3�֣�
��3����25��V��50��2�֣� ��60%��2�֣�
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 �ܼ�β���������ֲ��ػ�������Ҫ���ȵ������·��á����)��������������ÿ�������·������ĸB��C������������ѡ�õ�����(��������)���ױ�ʾ���£�
�ܼ�β���������ֲ��ػ�������Ҫ���ȵ������·��á����)��������������ÿ�������·������ĸB��C������������ѡ�õ�����(��������)���ױ�ʾ���£�
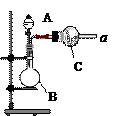


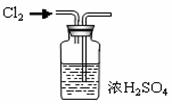
 ��Na2CO3��������A�м���ϡ���ᣬB�м���Na2CO3��NaCl�������C�м����ʯ�ҡ���װ�ô��ڽ϶�ȱ�ݣ��Ӷ�����ʵ�����ϴ�����˵�����е�����ȱ�ݣ�
��Na2CO3��������A�м���ϡ���ᣬB�м���Na2CO3��NaCl�������C�м����ʯ�ҡ���װ�ô��ڽ϶�ȱ�ݣ��Ӷ�����ʵ�����ϴ�����˵�����е�����ȱ�ݣ�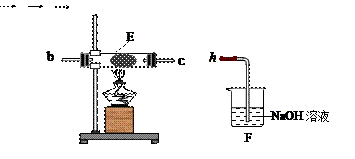 a b c h��
a b c h��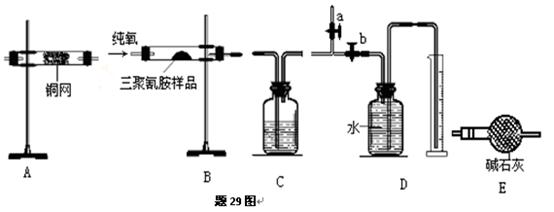
 �ζ�����̪��ָʾ�������յ�ʱ����
�ζ�����̪��ָʾ�������յ�ʱ���� �ζ�������
�������