��Ŀ����
��15�֣�
�����CO2�ڸ�������ľ̿��Ӧ����CO��ʵ�顣
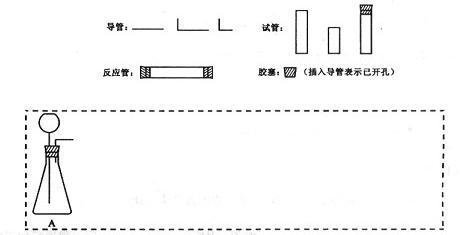
(1)�����淽���У�A��ʾ�г���©������ƿ��ɵ����巢���������ڴ���ϵ�A����ɸ÷�Ӧ��ʵ��װ��ʾ��ͼ(�г�װ��,���ӽ� �ܼ�β���������ֲ��ػ�������Ҫ���ȵ������·��á����)��������������ÿ�������·������ĸB��C������������ѡ�õ�����(��������)���ױ�ʾ���£�
�ܼ�β���������ֲ��ػ�������Ҫ���ȵ������·��á����)��������������ÿ�������·������ĸB��C������������ѡ�õ�����(��������)���ױ�ʾ���£�
(2)���ݷ����е�װ��ͼ���ڴ������д�ñ�
(3)���˶����巢���������¸Ľ�������ƿ�з���һС�Թܣ�������©���¶˲���С�Թ��С��Ľ�����ŵ���________________________��
(4)��֤CO�ķ�����____________________________��
�����CO2�ڸ�������ľ̿��Ӧ����CO��ʵ�顣
(1)�����淽���У�A��ʾ�г���©������ƿ��ɵ����巢���������ڴ���ϵ�A����ɸ÷�Ӧ��ʵ��װ��ʾ��ͼ(�г�װ��,���ӽ�
 �ܼ�β���������ֲ��ػ�������Ҫ���ȵ������·��á����)��������������ÿ�������·������ĸB��C������������ѡ�õ�����(��������)���ױ�ʾ���£�
�ܼ�β���������ֲ��ػ�������Ҫ���ȵ������·��á����)��������������ÿ�������·������ĸB��C������������ѡ�õ�����(��������)���ױ�ʾ���£�
(2)���ݷ����е�װ��ͼ���ڴ������д�ñ�
| �������� | �������������� | ���� |
| A | ʯ��ʯ��ϡ���� | ʯ��ʯ����������CO2 |
| | | |
(3)���˶����巢���������¸Ľ�������ƿ�з���һС�Թܣ�������©���¶˲���С�Թ��С��Ľ�����ŵ���________________________��
(4)��֤CO�ķ�����____________________________��
(1)
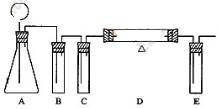
(2)
| ������� | �������������� | ���� |
| B | ����̼��������Һ | ��ȥCO2�е�HCl���� |
| C | Ũ���� | ��ȥCO2�е�ˮ�� |
| D | ����ľ̿�� | ��CO2��Ӧ����CO |
| E | ����ʯ��ˮ | ����δ��Ӧ��CO2 |
(3)ͨ����������������Ʋ�������Ŀ�����ͬʱС�Թ��г������ᣬ������Һ�����ã���ֹ������ʱ���徭©�������
��4����ȼ���壬�������ɫ������һ���ڱڸ��г���ʯ��ˮ���ձ����ڻ����ϣ��ձ��ڱڵ�ʯ��ˮ����ǡ�
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ


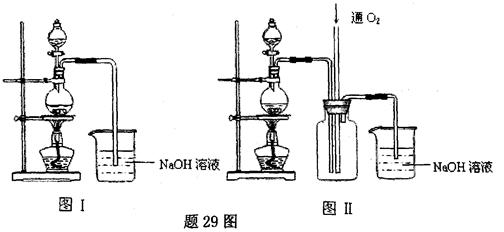
 ��2���������ɺ������Թ��е�����ˮ��Ϊ����ɫ����ʱ��������������ʹ����NaOH��Һ�������Թ��У������Թ����� ɫ�ij����������÷�Ӧ���뷽��ʽΪ ��
��2���������ɺ������Թ��е�����ˮ��Ϊ����ɫ����ʱ��������������ʹ����NaOH��Һ�������Թ��У������Թ����� ɫ�ij����������÷�Ӧ���뷽��ʽΪ ��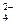 ��CO
��CO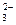 ��OH-�е�������ɣ����Ǿ����������ʣ���A������ˮ�������B������ˮ�����������ᣬ���ų���ɫ�̼�����ζ������E����C��ˮ��Һ�ʼ��ԣ������ᷴӦ����A����D������ˮ������������ʱ�ų�����E��E��ʹ����ʯ��ˮ����ǡ���ش�
��OH-�е�������ɣ����Ǿ����������ʣ���A������ˮ�������B������ˮ�����������ᣬ���ų���ɫ�̼�����ζ������E����C��ˮ��Һ�ʼ��ԣ������ᷴӦ����A����D������ˮ������������ʱ�ų�����E��E��ʹ����ʯ��ˮ����ǡ���ش�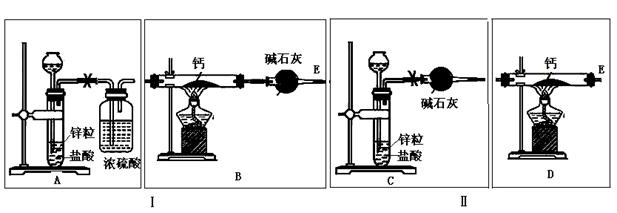
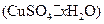 �����нᾧˮ�ĺ���ʱ������й��������±���
�����нᾧˮ�ĺ���ʱ������й��������±���
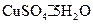 ���
��� �� ���ƫ�ߡ�����ƫ�͡��������䡱�������ܵ�ԭ���� ��������ĸ��ţ�
�� ���ƫ�ߡ�����ƫ�͡��������䡱�������ܵ�ԭ���� ��������ĸ��ţ�