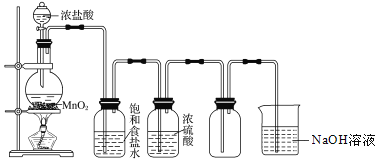
��Ŀ����
����Ŀ����ʳ�ִ���������(AlP)Ѭ��ɱ�棬AlP��ˮ������ǿ��ԭ�Ե�PH3���塣���ұ��涨��ʳ������(��PH3��)�IJ�����������0.05mg��kg��1ʱΪ�ϸ�ijС��ͬѧ��ͼ��ʾʵ��װ�ú�ԭ���ⶨij��ʳ��Ʒ������IJ�������C�м���100gԭ����E�м���20.00mL 2.50��10��4mol��L��1KMnO4��Һ(H2SO4�ữ)��C�м�������ˮ����ַ�Ӧ�����������Ʊ���Һ�ζ�E�е���Һ��
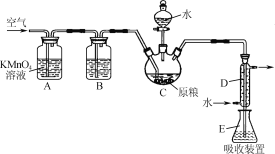
��1��װ��A�е�KMnO4��Һ��������________��
��2��װ��B��ʢװ����ûʳ����ļ�����Һ���տ����е�O2����ȥ����װ�ã����õ�����IJ�����________(����ƫ������ƫ��������������)��
��3��װ��E��PH3�����������ᣬMnO4-����ԭΪMn2����д���÷�Ӧ�����ӷ���ʽ��________��
��4���ռ�װ��E�е�����Һ����ˮϡ����250mL����ȡ���е�25.00mL����ƿ�У���4.0��10��5 mol��L��1��Na2SO3����Һ�ζ�������Na2SO3����Һ20.00 mL����Ӧԭ����SO32-��MnO4-��H����SO42-��Mn2����H2O(δ��ƽ)��ͨ�������жϸ���Ʒ�Ƿ�ϸ�(д���������)��_________
���𰸡����տ����еĻ�ԭ�����壬��ֹ�����pH3�IJⶨ ƫ�� 5PH3��8MnO4-��24H��===5H3PO4��8Mn2����12H2O 25mL��Һ��δ��Ӧ��n(KMnO4)��![]() ��4��10��5mol��L��1��0.020L��3.2��10��7mol��250mL��Һ��δ��Ӧ��n(KMnO4)��3.2��10��6mol����PH3��Ӧ��n(KMnO4)��0.02L��2.50��10��4 mol��L��1��3.2��10��6 mol��1.8��10��6 mol��n(PH3)��
��4��10��5mol��L��1��0.020L��3.2��10��7mol��250mL��Һ��δ��Ӧ��n(KMnO4)��3.2��10��6mol����PH3��Ӧ��n(KMnO4)��0.02L��2.50��10��4 mol��L��1��3.2��10��6 mol��1.8��10��6 mol��n(PH3)��![]() n(KMnO4)��
n(KMnO4)��![]() ��1.8��10��6 mol��1.125��10��6mol��100gԭ����m(PH3)��1.125��10��6 mol��34g��mol��1��3.825��10��5 g��1kgԭ��������������3.825��10��4 g��0.382 5 mg>0.05mg�����Բ��ϸ�
��1.8��10��6 mol��1.125��10��6mol��100gԭ����m(PH3)��1.125��10��6 mol��34g��mol��1��3.825��10��5 g��1kgԭ��������������3.825��10��4 g��0.382 5 mg>0.05mg�����Բ��ϸ�
��������
(1) ����PH3�����壬������װ���л�Ӱ����ʳ�в������ﺬ���IJⶨ������
(2) ��Ͽ����������������Ժ�PH3�Ļ�ԭ�Է�����
(3) ������Ŀ��֪��Ϣ����KMnO4�����Ի����г����Ļ�ԭ������õ���غ㡢�����غ㼰ԭ���غ���д��Ӧ�����ӷ���ʽ��
(4)����������ԭ��Ӧ�е����غ�����PH3�����������ò�Ʒ�ϸ�����жϼ��ɡ�
��1��װ��A�е�KMnO4��Һ����ǿ�����ԣ������������տ����еĻ�ԭ�����壬��ֹ�����pH3�IJⶨ��
��2��װ��B��ʢװ����ûʳ����ļ�����Һ���տ����е�O2����ȥ����װ�ã������е������ͻ�����PH3���壬���õ�����IJ�����ƫ�ͣ�
(3)��װ��E��PH3������KMnO4��Һ���������ᣬKMnO4 ����ԭΪMnSO4�� ͬʱ����ˮ�����ݵ���غ㡢�����غ㼰ԭ���غ㣬�ɵø÷�Ӧ�����ӷ���ʽΪ5PH3��8MnO4-��24H��=5H3PO4��8Mn2����12H2O��
��4��25mL��Һ��δ��Ӧ��n(KMnO4)��![]() ��4��10��5mol��L��1��0.020L��3.2��10��7mol��250mL��Һ��δ��Ӧ��n(KMnO4)��3.2��10��6mol����PH3��Ӧ��n(KMnO4)��0.02L��2.50��10��4 mol��L��1��3.2��10��6 mol��1.8��10��6 mol��n(PH3)��
��4��10��5mol��L��1��0.020L��3.2��10��7mol��250mL��Һ��δ��Ӧ��n(KMnO4)��3.2��10��6mol����PH3��Ӧ��n(KMnO4)��0.02L��2.50��10��4 mol��L��1��3.2��10��6 mol��1.8��10��6 mol��n(PH3)��![]() n(KMnO4)��
n(KMnO4)��![]() ��1.8��10��6 mol��1.125��10��6mol��100gԭ����m(PH3)��1.125��10��6 mol��34g��mol��1��3.825��10��5 g��1kgԭ��������������3.825��10��4 g��0.382 5 mg>0.05mg�����Բ��ϸ�
��1.8��10��6 mol��1.125��10��6mol��100gԭ����m(PH3)��1.125��10��6 mol��34g��mol��1��3.825��10��5 g��1kgԭ��������������3.825��10��4 g��0.382 5 mg>0.05mg�����Բ��ϸ�