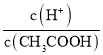��Ŀ����
����Ŀ�����дӺ����л�ȡ���ʵķ�������������
A��������ͨ�����ӡ����Ƶõ�����ʳ��ˮ��Ȼ����õ�����������ʯ���鷴Ӧ�Ƶ�Ư��
B����±�ữ��ͨ�������õ���ˮ��Ȼ�����ȿ�������ͨ�뵽SO2ˮ��Һ�У����ͨ������������ô���
C���ɺ������պ��ˮ��ȡ��Ȼ�����H2O2�õ���ˮ�����ͨ����ȡ����Һ������õ��ⵥ��
D����ˮ�м���ʯ����õ�Mg(OH)2����������õ�MgCl2��Һ����������Һ�õ�þ����
���𰸡�D
��������
���������A�������к�����ɳ��Ca2+��Mg2+��SO42�������ʣ������μ���BaCl2��Na2CO3��NaOH��ȥSO42����Ca2+��Mg2+���ٹ��˳�ȥ��ɳ��������������ϡ�������pH����ȥ������Na2CO3��NaOH���Ӷ��õ����Ʊ���ʳ��ˮ��Ȼ����õ�����������2NaCl+2H2O![]() 2NaOH+H2��+Cl2��������Cl2��ʯ���鷴Ӧ�Ƶ�Ư�ۣ�����2Cl2+2Ca(OH)2��CaCl2+Ca(ClO)2+2H2O����A��ȷ��B����±�к���Br�����ữ��ͨ�������õ���ˮ��Cl2+2Br����2Cl��+Br2����Ȼ�����ȿ�������ͨ�뵽SO2ˮ��Һ�У�Br2+SO2+2H2O��H2SO4+2HBr�������ͨ������������ô��壨2HBr+Cl2��2HCl+Br2������B��ȷ��C���ɺ������պ��ˮ��ȡ��Ȼ�����H2O2�õ���ˮ�����ͨ����ȡ����Һ������õ��ⵥ�ʣ���C��ȷ��
2NaOH+H2��+Cl2��������Cl2��ʯ���鷴Ӧ�Ƶ�Ư�ۣ�����2Cl2+2Ca(OH)2��CaCl2+Ca(ClO)2+2H2O����A��ȷ��B����±�к���Br�����ữ��ͨ�������õ���ˮ��Cl2+2Br����2Cl��+Br2����Ȼ�����ȿ�������ͨ�뵽SO2ˮ��Һ�У�Br2+SO2+2H2O��H2SO4+2HBr�������ͨ������������ô��壨2HBr+Cl2��2HCl+Br2������B��ȷ��C���ɺ������պ��ˮ��ȡ��Ȼ�����H2O2�õ���ˮ�����ͨ����ȡ����Һ������õ��ⵥ�ʣ���C��ȷ��
D����ˮ�м���ʯ����õ�Mg(OH)2����������õ�MgCl2��Һ��Ȼ����HCl�����м������ɵ���ˮMgCl2�����������MgCl2�õ�þ���ʣ���D����ѡD��

 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�����Ŀ����֪![]() ʱ��
ʱ��![]() ��HCN��
��HCN��![]() �ĵ��볣�������
�ĵ��볣�������
| HCN |
|
|
|
|
����������������![]()
A.��NaCN��Һ��ͨ������![]() �����ӷ���ʽ��
�����ӷ���ʽ��![]()
B.![]() ʱ����Ӧ
ʱ����Ӧ![]() �Ļ�ѧƽ�ⳣ��Ϊ
�Ļ�ѧƽ�ⳣ��Ϊ![]()
C.�к͵�����������ʵ���Ũ�ȵ�![]() ��HCN��Һ������NaOH����ǰ��С�ں���
��HCN��Һ������NaOH����ǰ��С�ں���
D.�����ʵ���Ũ�ȵ�![]() ��NaCN�����Һ�У�
��NaCN�����Һ�У�![]()