��Ŀ����
 ��98%��ŨH2SO4����=1.84g/cm3������480mL0.5mol/L��ϡH2SO4���밴Ҫ����գ�
��98%��ŨH2SO4����=1.84g/cm3������480mL0.5mol/L��ϡH2SO4���밴Ҫ����գ�
��1������ŨH2SO4�����ʵ���Ũ��Ϊ______������ʱ����Ũ��������Ϊ______mL����������һλС����
��2��ʵ������Ҫ�õ��Ķ���������______��
��3��ʵ���в�����������______
��4����ʵ���г�����������������Һ��Ũ����ʲôӰ�죿���ƫ�ߡ�����ƫ�͡�����Ӱ�족��
������Ͳ��ȡ����Ũ���ᵹ���ձ�������ˮϴ��Ͳ2��3�Σ�ϴҺ�����ձ���______
��Ũ�����ܽ��δ�������¼�����ת�ơ����ݣ�______
�۶���ʱ���ӿ̶��ߣ�______
��5��������ʱҺ����ڿ̶���Ӧ��ȡ�Ĵ�ʩ��______��
��6����ʵ�����ȫ������������õ���ҺӦ������Լ�ƿ�У������ϱ�ǩ����������ѱ�ǩ�ϵ�����дһ�£�����ͼ��______��
�⣺��1��ŨH2SO4�����ʵ���Ũ��c=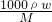 =
= mol/L=18.4mol/L��
mol/L=18.4mol/L��
����480mL0.2mol/L��ϡH2SO4����ѡ��500mL����ƿ������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL��18.4mol/L=500mL��0.2mol/L����ã�x��5.4������Ӧ��ȡ��Ũ���������5.4mL���ʴ�Ϊ��18.4mol/L�� 5.4��
��2�����Ʋ�������ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ������Ͳ��ȡ���õ���ͷ�ιܣ����������ձ���ϡ�ͣ���ȴ��ת�Ƶ�500mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ�����������ձ�����ͷ�ιܡ�10 mL��Ͳ��500mL����ƿ���ʴ�Ϊ��10 mL��Ͳ��500mL����ƿ��
��3�����ܽ������Ϊ�˼ӿ��ܽ⣬�ò��������裬��Һ������Ϊ��ʹ��Һȫ��ת������ƿ���ò������������ʴ�Ϊ�����衢������
��4��������Ͳ��ȡ����Ũ���ᵹ���ձ�������ˮϴ��Ͳ2��3�Σ�ϴҺ�����ձ��У�Ũ���������ƫ�࣬���ʵ���ƫ�࣬Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��Ũ�����ܽ��δ�������¼�����ת�ơ����ݣ�һ����ȴ�����ᵼ�����ƫС��Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
�۶���ʱ���ӿ̶��ߣ���Һ���ƫ��Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
��5����ʵ�����������һ�����ִ�������������ƣ��ʴ�Ϊ���������ƣ�
��6����ǩ�ϵ�����Ӧд�����ʵ����ƺ�Ũ�ȣ��ʴ�Ϊ�� ��
��
��������1������ŨH2SO4�����ʵ���Ũ��c=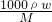 ��������Һϡ��ǰ�����ʵ��������������Ũ����������
��������Һϡ��ǰ�����ʵ��������������Ũ����������
��2������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������
��3�����ݲ��������ܽ����Һ�����е����ã�
��4������c=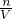 �������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��5��ʵ�����������һ�����ִ�������������ƣ�
��6�����ݱ�ǩӦע�����ʵ����ƺ�Ũ�ȣ�
���������⿼����һ�����ʵ���Ũ����Һ�����Ƶ���������ע���c=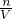 �������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
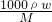 =
= mol/L=18.4mol/L��
mol/L=18.4mol/L������480mL0.2mol/L��ϡH2SO4����ѡ��500mL����ƿ������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL��18.4mol/L=500mL��0.2mol/L����ã�x��5.4������Ӧ��ȡ��Ũ���������5.4mL���ʴ�Ϊ��18.4mol/L�� 5.4��
��2�����Ʋ�������ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ������Ͳ��ȡ���õ���ͷ�ιܣ����������ձ���ϡ�ͣ���ȴ��ת�Ƶ�500mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ�����������ձ�����ͷ�ιܡ�10 mL��Ͳ��500mL����ƿ���ʴ�Ϊ��10 mL��Ͳ��500mL����ƿ��
��3�����ܽ������Ϊ�˼ӿ��ܽ⣬�ò��������裬��Һ������Ϊ��ʹ��Һȫ��ת������ƿ���ò������������ʴ�Ϊ�����衢������
��4��������Ͳ��ȡ����Ũ���ᵹ���ձ�������ˮϴ��Ͳ2��3�Σ�ϴҺ�����ձ��У�Ũ���������ƫ�࣬���ʵ���ƫ�࣬Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��Ũ�����ܽ��δ�������¼�����ת�ơ����ݣ�һ����ȴ�����ᵼ�����ƫС��Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
�۶���ʱ���ӿ̶��ߣ���Һ���ƫ��Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
��5����ʵ�����������һ�����ִ�������������ƣ��ʴ�Ϊ���������ƣ�
��6����ǩ�ϵ�����Ӧд�����ʵ����ƺ�Ũ�ȣ��ʴ�Ϊ��
 ��
����������1������ŨH2SO4�����ʵ���Ũ��c=
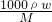 ��������Һϡ��ǰ�����ʵ��������������Ũ����������
��������Һϡ��ǰ�����ʵ��������������Ũ������������2������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������
��3�����ݲ��������ܽ����Һ�����е����ã�
��4������c=
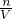 �������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
�������������ʵ����ʵ��������Һ�������Ӱ���жϣ���5��ʵ�����������һ�����ִ�������������ƣ�
��6�����ݱ�ǩӦע�����ʵ����ƺ�Ũ�ȣ�
���������⿼����һ�����ʵ���Ũ����Һ�����Ƶ���������ע���c=
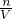 �������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
�������������ʵ����ʵ��������Һ�������Ӱ���жϣ� 
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��98%��ŨH2SO4����=1.84g/cm3������480mL0.5mol/L��ϡH2SO4���밴Ҫ����գ�
��98%��ŨH2SO4����=1.84g/cm3������480mL0.5mol/L��ϡH2SO4���밴Ҫ����գ� ��98%��ŨH2SO4����=1.84g/cm3������480mL0.5mol/L��ϡH2SO4���밴Ҫ����գ�
��98%��ŨH2SO4����=1.84g/cm3������480mL0.5mol/L��ϡH2SO4���밴Ҫ����գ�