��Ŀ����
��ҵ�������β���к���һ����SO2������β�����ֶγ��˰����շ���������¼��ַ�������һ����ҵʵ�����չ����У���I��������Ũ��������Һ����Ϊ���е�SO2��Ȼ����������Һ�м�����ʯ�ң���ַ�Ӧ�����ɲ��������پ��������Ƶò�ƷA��
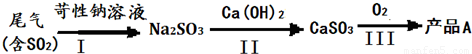
��1����ƷA��______���ѧʽ����
��2�������������һ���е�Ũ��������Һ����ͬ�¶��±���Ca��OH��2��Һֱ���Ƶò�ƷCaSO3������Ϊ�Ƿ���У�______������ԡ����������ԡ���ȷ������ԭ����______
�������Ʊ�MnSO4?H2O��SO2��ԭMnO2���Ʊ�MnSO4?H2O���������£�
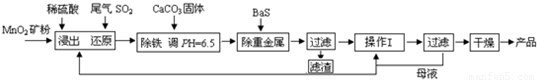
��1�����������MnO2������ʵĴ�ʩ���˽���ʯ���黹��______�����һ�����ɣ���
��2���������������ӷ�Ӧ����ʽΪ______��
��3������I������Ϊ______��
��4��MnSO4?H2O��1150��������ֽ⣬������Mn3O4�������ˮ���ڸ������������̾���ֽⷴӦ�Ļ�ѧ����ʽ��______��
��2��Ũ��������Һ����ͬ�¶��±���Ca��OH��2��Һֱ���Ƶò�ƷCaSO3ʱ�����������ܽ�Ƚ�С�����ն��������Ч�ʲ��߷����жϣ�
�����������Ʊ�MnSO4?H2O��ԭ�������̷�����SO2��ԭMnO2���Ʊ�MnSO4?H2O��
��1�����������MnO2������ʵĴ�ʩ���˽���ʯ���飬������ʩ���ܼӿ��ܽ����ʷ�����
��2�����������ķ�Ӧ����������������ˮ���������������������ӳ����ԣ�����PH��6.5 ʱ��������ȫ�������������̼�����Ϊ�˺������ӷ�Ӧʹ��ҺPH���ߵ�6.5��
��3���������̷���������I�ǶԵõ�����������Һ�ᾧ����������Ϊ�����ܼ�Ũ����ȴ�ᾧ��
��4��MnSO4?H2O��1150��������ֽ⣬������Mn3O4�������ˮ�����ݻ��ϼ۵�������ͬ��ԭ���غ���д��ѧ����ʽ��
����⣺��һ����1�����̷����ķ�Ӧ�Ƕ���������������Ʒ�Ӧ�����������ƣ��������ƺ��������Ʒ�Ӧ����������ƣ�������Ʊ���������Ϊ����ƣ���ѧʽΪ��CaSO4��
�ʴ�Ϊ��CaSO4��
��2������һ���е�Ũ��������Һ����ͬ�¶��±���Ca��OH��2��Һֱ���Ƶò�ƷCaSO3���������������ܽ��С�Զ����������ղ���ȫ�����Բ����ԣ�
�ʴ�Ϊ�������� Ca��OH��2��ˮ���ܽ�Ƚ�С��c��OH-��̫�ͣ�����Ч�ʲ��ߣ�
��������1�����������MnO2������ʵĴ�ʩ���˽���ʯ���飬Ҳ���Խ��г�ֽ��衢�ʵ������ϵ�¶ȵȴ�ʩ���ʴ�Ϊ����ֽ��衢�ʵ������ϵ�¶ȵȣ�
��2�����������ķ�Ӧ��������������PH=6.5ʱȫ��������ȥ������������ˮ���������������������ӣ�����̼��ƻ�������ӷ�Ӧ��ʹPH���ߵ�6.5�����Է�Ӧ�����ӷ�Ӧ����ʽΪ��2Fe3++3H2O+3CaCO3=2Fe��OH��3��+3CO2��+3Ca2+���� Fe3++3H2O
 Fe��OH��3+3H+�� 2H++CaCO3=Ca2++CO2��+H2O��
Fe��OH��3+3H+�� 2H++CaCO3=Ca2++CO2��+H2O���ʴ�Ϊ��2Fe3++3H2O+3CaCO3=2Fe��OH��3��+3CO2��+3Ca2+���� Fe3++3H2O
 Fe��OH��3+3H+�� 2H++CaCO3=Ca2++CO2��+H2O��
Fe��OH��3+3H+�� 2H++CaCO3=Ca2++CO2��+H2O����3������I���������̵õ���������Һ�еõ����ʾ��壬��ȡ�Ĵ�ʩ�ǣ�����Ũ������ȴ�ᾧ���ʴ�Ϊ������Ũ������ȴ�ᾧ��
��4��MnSO4?H2O��1150��������ֽ⣬������Mn3O4�������ˮ���ڸ������������̾���ֽⷴӦ�Ļ�ѧ����ʽ����������ԭ��Ӧ�Ļ��ϼ�������ͬ�����ԭ���غ�����ж�д����ѧ����ʽΪ�����ݵ����غ��֪��3MnSO4?H2O��Mn3O4��2e-�� 3MnSO4?H2O��SO2��6e-�� �������ɵ���Ļ������Ƕ��������������������ԭ���غ���ƽ��ѧ����ʽΪ��3MnSO4?H2O
 Mn3O4+SO2��+2SO3��+3H2O��
Mn3O4+SO2��+2SO3��+3H2O���ʴ�Ϊ��3MnSO4?H2O
 Mn3O4+SO2��+2SO3��+3H2O��
Mn3O4+SO2��+2SO3��+3H2O�����������⿼���������Ʊ������̷��������ӷ���ʽ����д��������ԭ��Ӧ�ĵ����غ�ķ���Ӧ�ã�ע�������е�ʵ������жϺͲ���ķ����Ǹ��ݣ��Ѷ��еȣ�

ʵ����� | �� | �� | �� | �� |
������Һ�����/Ml | 50 | 50 | 50 | 50 |
�����/g | 9.260 | 13.890 | 20.835 | 32.410 |
������������/mL | 1 344 | 2 016 | 3 024 | 2 464 |
(1)��������ʵ���У�SO2�������������֮����ͬ�������_______________���ɵڢ���ʵ���е�SO2�������������֮�ȣ�����������6.945 g�ù������ͬ����ʵ��ʱ������SO2__________________mL(��״��)�����ݱ���__________________�������ݱ仯��ȷ�ϸù�����һ������(NH4)2SO3��
(2)��ȡ9.260 g�ù�������������ʯ�ҹ��ȣ��ռ�����״���İ��������Ϊ3136 mL����ù���ijɷֳ�(NH4)2SO3���__________________ (�ѧʽ)����ʵ����ʹ�õ���������ʵ���Ũ��Ϊ__________________��
��15�֣���ҵ�������β���к��϶��SO2��Ϊ��ֹ��Ⱦ��������������SO2����ҵ�ϳ��ð�ˮ���շ�����β����ijУ��ѧ��ȤС���ͬѧΪȷ���÷������ù���ijɷ֣���ȡ�ù����ķݣ�����ˮ�ֱ���μ�����ͬŨ�ȵ�������Һ50 mL������SO2���������״�������±���ʵ��ʱ�跨ʹˮ���ܽ��SO2������ȫ�ݳ�����
| ʵ����� | �� | �� | �� | �� |
| ������Һ�������mL�� | 50 | 50 | 50 | 50 |
| �������g�� | 9.260 | 13.890 | 20.835 | 32.410 |
| ��������������mL�� | 1344 | 2016 | 3024 | 2464 |
����ȡ9.260 g�ù�������������ʯ�ҹ��ȣ��ռ�����״���İ��������Ϊ3136 mL����ù���ijɷֳ�(NH4)2SO3���___________���ѧʽ������ʵ����ʹ�õ���������ʵ���Ũ��Ϊ_______________��
��15�֣���ҵ�������β���к��϶��SO2��Ϊ��ֹ��Ⱦ��������������SO2����ҵ�ϳ��ð�ˮ���շ�����β����ijУ��ѧ��ȤС���ͬѧΪȷ���÷������ù���ijɷ֣���ȡ�ù����ķݣ�����ˮ�ֱ���μ�����ͬŨ�ȵ�������Һ50 mL������SO2���������״�������±���ʵ��ʱ�跨ʹˮ���ܽ��SO2������ȫ�ݳ�����
|
ʵ����� |
�� |
�� |
�� |
�� |
|
������Һ�������mL�� |
50 |
50 |
50 |
50 |
|
�������g�� |
9.260 |
13.890 |
20.835 |
32.410 |
|
��������������mL�� |
1344 |
2016 |
3024 |
2464 |
����������ʵ���У�SO2�������������֮����ͬ�������__________________���ɵڢ���ʵ���е�SO2�������������֮�ȣ�����������6.945 g�ù������ͬ����ʵ��ʱ������SO2_________mL(��״��)�����ݱ��� �������ݱ仯��ȷ�ϸù�����һ������(NH4)2SO3��
����ȡ9.260 g�ù�������������ʯ�ҹ��ȣ��ռ�����״���İ��������Ϊ3136 mL����ù���ijɷֳ�(NH4)2SO3���___________���ѧʽ������ʵ����ʹ�õ���������ʵ���Ũ��Ϊ_______________��
��ҵ�������β���к��϶��SO2��Ϊ��ֹ��Ⱦ��������������SO2����ҵ�ϳ��ð�ˮ���շ�����β����ijУ��ѧ��ȤС���ͬѧΪȷ���÷������ù���ijɷ֣���ȡ�ù����ķݣ�����ˮ�ֱ���μ�����ͬŨ�ȵ�������Һ50mL������SO2���������״�������±���ʵ��ʱ�跨ʹˮ���ܽ��SO2������ȫ�ݳ�����
| ʵ����� | �� | �� | �� | �� |
| ������Һ�������mL�� | 50 | 50 | 50 | 50 |
| �������g�� | 9.260 | 13.890 | 20.835 | 32.410 |
| ��������������mL�� | 1344 | 2016 | 3024 | 2464 |
��2����ȡ9.260g�ù�������������ʯ�ҹ��ȣ��ռ�����״���İ��������Ϊ3136mL����ù���ijɷֳ���NH4��2SO3���______���ѧʽ������ʵ����ʹ�õ���������ʵ���Ũ��Ϊ______��