��Ŀ����
��15�֣���ҵ�������β���к��϶��SO2��Ϊ��ֹ��Ⱦ��������������SO2����ҵ�ϳ��ð�ˮ���շ�����β����ijУ��ѧ��ȤС���ͬѧΪȷ���÷������ù���ijɷ֣���ȡ�ù����ķݣ�����ˮ�ֱ���μ�����ͬŨ�ȵ�������Һ50 mL������SO2���������״�������±���ʵ��ʱ�跨ʹˮ���ܽ��SO2������ȫ�ݳ�����
|
ʵ����� |
�� |
�� |
�� |
�� |
|
������Һ�������mL�� |
50 |
50 |
50 |
50 |
|
�������g�� |
9.260 |
13.890 |
20.835 |
32.410 |
|
��������������mL�� |
1344 |
2016 |
3024 |
2464 |
����������ʵ���У�SO2�������������֮����ͬ�������__________________���ɵڢ���ʵ���е�SO2�������������֮�ȣ�����������6.945 g�ù������ͬ����ʵ��ʱ������SO2_________mL(��״��)�����ݱ��� �������ݱ仯��ȷ�ϸù�����һ������(NH4)2SO3��
����ȡ9.260 g�ù�������������ʯ�ҹ��ȣ��ռ�����״���İ��������Ϊ3136 mL����ù���ijɷֳ�(NH4)2SO3���___________���ѧʽ������ʵ����ʹ�õ���������ʵ���Ũ��Ϊ_______________��
�Ţ٢ڢۣ�1008 mL���ۢܣ���ÿ��3�֣���9�֣�
��(NH4)2SO4��NH4HSO3�� 2.5 mol•L-1����6�֣�
����������1�����ݱ������ݿ�֪�٢ڢ���SO2�������������֮����ͬ�����Ǹ��ݵڢ���ʵ���е�SO2�������������֮�ȿ�֪��6.945 g�ù������ɵ�SO2��6.945 g/9.260g��1344ml��1008 mL�����ݢۢܿ�֪�����ӹ������������������SO2�������٣���˵��������һ������������李���������������ᷴӦ�Ƿֲ����еģ������㣬�����ɵ�SO2���١�
��2���ⷨһ����9.260 g������(NH4)2SO3��NH4HSO3��(NH4)2SO4�����ʵ����ֱ�Ϊx��y��z
�� x �� y = 0.06 2x �� y ��z = 0.14 116x �� 99y �� 132z = 9.26 g
��ã�x = 0.04 mol y = 0.02 mol z=0.02 mol
�ڢ�����������ǵڢ����������3.5������ڢ��������(NH4)2SO3��NH4HSO3��(NH4)2SO4�����ʵ����ֱ�Ϊ0.14 mol��0.07 mol��0.07 mol
���ĵ���������ʵ�����n(H2SO4)=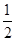 [n((NH4)2SO3)+n(SO2)]=
[n((NH4)2SO3)+n(SO2)]=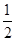 (0.14
mol+
(0.14
mol+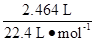 )=0.125
mol
)=0.125
mol
��c(H2SO4)= 2.5 mol•L-1
�ⷨ�����ڢ���������һ��û��ʣ�࣬��Ӧ��������Һ�к�(NH4)2SO4��NH4HSO3
�ڢ����й��庬SΪ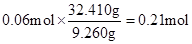 ��NH4+��0.14 mol��3.5 = 0.49 mol
��NH4+��0.14 mol��3.5 = 0.49 mol
��Ӧ��������Һ�к�NH4HSO3Ϊ��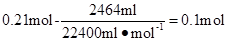
��ݵ���غ�ã�������Һ�к�SO42�������ʵ���Ϊ����0.49 mol-0.1 mol��/2=0.195 mol
ԭ�����к�(NH4)2SO4��0.02 mol��3.5 = 0.07 mol
��H2SO4�����ʵ���Ϊ0.195 mol��0.07 mol = 0.125 mol ��c(H2SO4)= 2.5 mol•L-1�����������ⷨ�����֣�

ʵ����� | �� | �� | �� | �� |
������Һ�����/Ml | 50 | 50 | 50 | 50 |
�����/g | 9.260 | 13.890 | 20.835 | 32.410 |
������������/mL | 1 344 | 2 016 | 3 024 | 2 464 |
(1)��������ʵ���У�SO2�������������֮����ͬ�������_______________���ɵڢ���ʵ���е�SO2�������������֮�ȣ�����������6.945 g�ù������ͬ����ʵ��ʱ������SO2__________________mL(��״��)�����ݱ���__________________�������ݱ仯��ȷ�ϸù�����һ������(NH4)2SO3��
(2)��ȡ9.260 g�ù�������������ʯ�ҹ��ȣ��ռ�����״���İ��������Ϊ3136 mL����ù���ijɷֳ�(NH4)2SO3���__________________ (�ѧʽ)����ʵ����ʹ�õ���������ʵ���Ũ��Ϊ__________________��
��15�֣���ҵ�������β���к��϶��SO2��Ϊ��ֹ��Ⱦ��������������SO2����ҵ�ϳ��ð�ˮ���շ�����β����ijУ��ѧ��ȤС���ͬѧΪȷ���÷������ù���ijɷ֣���ȡ�ù����ķݣ�����ˮ�ֱ���μ�����ͬŨ�ȵ�������Һ50 mL������SO2���������״�������±���ʵ��ʱ�跨ʹˮ���ܽ��SO2������ȫ�ݳ�����
| ʵ����� | �� | �� | �� | �� |
| ������Һ�������mL�� | 50 | 50 | 50 | 50 |
| �������g�� | 9.260 | 13.890 | 20.835 | 32.410 |
| ��������������mL�� | 1344 | 2016 | 3024 | 2464 |
����ȡ9.260 g�ù�������������ʯ�ҹ��ȣ��ռ�����״���İ��������Ϊ3136 mL����ù���ijɷֳ�(NH4)2SO3���___________���ѧʽ������ʵ����ʹ�õ���������ʵ���Ũ��Ϊ_______________��
��ҵ�������β���к��϶��SO2��Ϊ��ֹ��Ⱦ��������������SO2����ҵ�ϳ��ð�ˮ���շ�����β����ijУ��ѧ��ȤС���ͬѧΪȷ���÷������ù���ijɷ֣���ȡ�ù����ķݣ�����ˮ�ֱ���μ�����ͬŨ�ȵ�������Һ50mL������SO2���������״�������±���ʵ��ʱ�跨ʹˮ���ܽ��SO2������ȫ�ݳ�����
| ʵ����� | �� | �� | �� | �� |
| ������Һ�������mL�� | 50 | 50 | 50 | 50 |
| �������g�� | 9.260 | 13.890 | 20.835 | 32.410 |
| ��������������mL�� | 1344 | 2016 | 3024 | 2464 |
��2����ȡ9.260g�ù�������������ʯ�ҹ��ȣ��ռ�����״���İ��������Ϊ3136mL����ù���ijɷֳ���NH4��2SO3���______���ѧʽ������ʵ����ʹ�õ���������ʵ���Ũ��Ϊ______��