��Ŀ����
����Ŀ��������������ȷ����
![]() A. ����CaCO3������ˮ��ͨ��CO2 ������ǡ���ܽ⣬������Һ�м���NaHCO3 ��
A. ����CaCO3������ˮ��ͨ��CO2 ������ǡ���ܽ⣬������Һ�м���NaHCO3 ��
����Һ������CaCO3��������
B. ��Na2 CO3��Һ����μ�������ʵ�����ϡ���ᣬ���ɵ�CO2��ԭNa2 CO3�����ʵ���֮��Ϊ1��2.
C. ��������NaHCO3��Na2 CO3�ֱ����������ᷴӦ����ͬ��ͬѹ�£����ɵ�CO2�����ͬ
D. ��Na2 CO3������Һ��ͨ��CO2����NaHCO3�ᾧ����
���𰸡�D
��������CaCO3��CO2��Ӧ����Ca(HCO3)2,�ټ���NaHCO3��û������ģ�A�����
��Na2CO3��Һ����μ�������ʵ�����ϡ���ᣬ������NaHCO3����CO2����ų���B�����
��������NaHCO3��Na2CO3������NaHCO3�����ʵ����࣬������HCl��Ӧʱ���ų���CO2�࣬C�����
D������ķ�ӦΪ��Na2CO3 + CO2 + H2O =2NaHCO3��������NaHCO3���ܽ��Խ�С�����нᾧ��������ȷ��

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�����Ŀ��������һ�ֳ����Ļ������ҵ����Ҳ��һ����ȡ������ͭ�������ԭ�ϡ����з�ͭ����Ҫ����ΪFe�����Ʊ�����������������������̣�
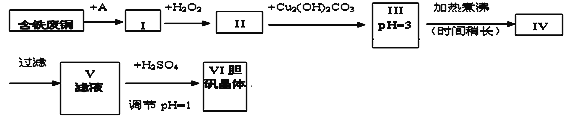
pHֵ���ƿɲο���������
�� �� | ��ʼ����ʱ��pHֵ | ��ȫ����ʱ��pHֵ |
�������� | 2.7 | 3.7 |
���������� | 7.6 | 9.6 |
������ͭ | 5.2 | 6.4 |
������������̻ش��������⣺
��1��A���ʿ�ѡ��_____������ĸ����
a��ϡH2SO4 b��ŨH2SO4/�� c��ŨFeCl3��Һ d��ŨHNO3
��2��I�м�H2O2�����ӷ���ʽ________________________________________��
��3��II�м�Cu2��OH��2CO3��Ŀ����________________________�����ŵ���__________��
��4��III�������ʱ�����Ļ�ѧ��Ӧ�����ӷ���ʽΪ________________________��
��5��V�м�H2SO4����pH=1��Ϊ��____________________________________________��
��6��V��VI�IJ�����_________________________________
��7��ijͬѧ��Ϊ�������������ӵ�A���ʲ������룬�����Ľ�����������__________����θĽ�___________________��
