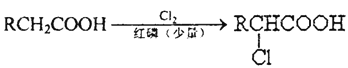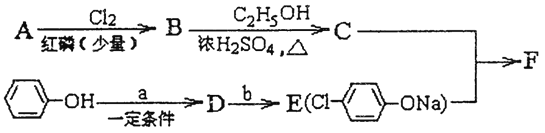��Ŀ����
����Ŀ����ˮ��ɽ���ǽ�ɽ��ɽ���ҹ�������Ա��̽����ν��ʹ����е���������̼������ĺ������������˾��ף���ȡ���������ijɼ���
(1)���й��ڵ���������̼�������˵������ȷ����_____(����ĸ)��
A.CO2��NO2����������������
B.NO��CO���������ſ������ռ�
C.��ȥNO�л��е�NO2�ķ����ǽ��������ͨ����������������Һ��
D.��ȥCO�л��е�CO2�ķ����ǽ��������ͨ����������������Һ��
(2)��֪:N2(g)+O2(g)![]() 2NO(g)��H1=+180.5kJ/mol;
2NO(g)��H1=+180.5kJ/mol;
CO(g)![]() C(s)+1/2O2(g)��H2=+110.5kJ/mol;
C(s)+1/2O2(g)��H2=+110.5kJ/mol;
C(s)+O2(g)![]() CO2(g)��H3=-393.5kJ/mol��
CO2(g)��H3=-393.5kJ/mol��
��Ӧ2NO(g)+2CO(g)![]() N2(g)+2CO2(g)��H=__________
N2(g)+2CO2(g)��H=__________
(3)��Ӧ2NO(g)+2CO(g)![]() 2CO2(g)+N2(g)�����ھ�������β������֪570Kʱ�÷�Ӧ�ķ�Ӧ���ʼ���,ƽ�ⳣ�������ɴ˿�֪�����β������Ч�ʵ����;����_________;��Ҫ��������β����ͬʱ��߸÷�Ӧ�ķ�Ӧ���ʺ�NO��ת����,��ֻ�ı�һ����Ӧ����,��Ӧ��ȡ�Ĵ�ʩ��_________��
2CO2(g)+N2(g)�����ھ�������β������֪570Kʱ�÷�Ӧ�ķ�Ӧ���ʼ���,ƽ�ⳣ�������ɴ˿�֪�����β������Ч�ʵ����;����_________;��Ҫ��������β����ͬʱ��߸÷�Ӧ�ķ�Ӧ���ʺ�NO��ת����,��ֻ�ı�һ����Ӧ����,��Ӧ��ȡ�Ĵ�ʩ��_________��
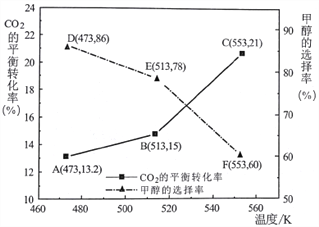
(4)ij����С����ݷ�Ӧ2NO(g)+2CO(g)![]() N2(g)+2CO2(g)��̽����ʼ��Ӧ���̼����[n(CO)/n(NO)]����Ⱦ��ȥ���ʵ�Ӱ�졣T��ʱ�������Ϊ1L�ĺ����ܱ������г��������ʵ���Ϊ4mol��NO��CO������壬������һ�����Ĺ���������з�Ӧ,ʵ����ƽ����ϵ��������ֵ�ת���ʺ���������������ı仯��ͼ��ʾ.
N2(g)+2CO2(g)��̽����ʼ��Ӧ���̼����[n(CO)/n(NO)]����Ⱦ��ȥ���ʵ�Ӱ�졣T��ʱ�������Ϊ1L�ĺ����ܱ������г��������ʵ���Ϊ4mol��NO��CO������壬������һ�����Ĺ���������з�Ӧ,ʵ����ƽ����ϵ��������ֵ�ת���ʺ���������������ı仯��ͼ��ʾ.

�ٸ���ͼ���Ʋ�����ת����1��ʾ����______ (�CO����NO��)��
��A��ʱ��n(CO)/n(NO)=_________,��ʱ��Ӧ��ƽ�ⳣ��K= __________(����д��ֵ�뵥λ)��
(5)ú̿��O2/CO2��������ȼ�ջ����CO,�������,������Ʒ�Ӧ2CO(g)=2C(s)+O2(g)������CO����Ⱦ��������_______(����С������С�), ������_______��
���𰸡� BD -746.5k]/mol ���Ƹ�Ч���� ����ѹǿ NO 1 80L/mol ������ �÷�Ӧ��H>0����S<0,��H-T��S>0,�÷�Ӧ���κ�����¶������Է�����
��������(1) A.CO2�������������NO2��ˮ��Ӧ���������һ�����������������������ѡ��A����B.NO����������Ӧ��CO�ܶ���������ܶȽӽ������������ſ������ռ���ѡ��B��ȷ��C. NO��NO2�����������ܷ�Ӧ����ȥNO�л��е�NO2�ķ����ǽ��������ͨ��ˮ�У�ѡ��C����D.��ȥCO�л��е�CO2�ķ����ǽ��������ͨ����������������Һ�У�������̼���������Ʒ�Ӧ��һ����̼����Ӧ��ѡ��D��ȷ����ѡBD��(2)��֪:��N2(g)+O2(g)![]() 2NO(g)��H1=+180.5kJ/mol����CO(g)
2NO(g)��H1=+180.5kJ/mol����CO(g)![]() C(s)+1/2O2(g)��H2=+110.5kJ/mol����C(s)+O2(g)
C(s)+1/2O2(g)��H2=+110.5kJ/mol����C(s)+O2(g)![]() CO2(g)��H3=-393.5kJ/mol�����ݸ�˹���ɣ���
CO2(g)��H3=-393.5kJ/mol�����ݸ�˹���ɣ���![]() ��+�۵÷�Ӧ2NO(g)+2CO(g)
��+�۵÷�Ӧ2NO(g)+2CO(g)![]() N2(g)+2CO2(g)��H=2��H2-��H1+��H3=2
N2(g)+2CO2(g)��H=2��H2-��H1+��H3=2![]() 110.5kJ/mol-180.5kJ/mol-393.5kJ/mol=-746.5k]/mol����3�����Ƹ�Ч��������߷�Ӧ���ʣ������Ӧ���������⣬������β����ת��������ѹǿ����ѧ��Ӧ���ʼӿ죬��ѧƽ�������������С�ķ����ƶ���������Ӧ����NO��ת����ת��������(4)����ʼ��Ӧ���̼����[n(CO)/n(NO)]Խ��һ��������ת����Խ����ͼ���Ʋ�����ת����1��ʾ����NO���ڷ�Ӧ2NO(g)+2CO(g)
110.5kJ/mol-180.5kJ/mol-393.5kJ/mol=-746.5k]/mol����3�����Ƹ�Ч��������߷�Ӧ���ʣ������Ӧ���������⣬������β����ת��������ѹǿ����ѧ��Ӧ���ʼӿ죬��ѧƽ�������������С�ķ����ƶ���������Ӧ����NO��ת����ת��������(4)����ʼ��Ӧ���̼����[n(CO)/n(NO)]Խ��һ��������ת����Խ����ͼ���Ʋ�����ת����1��ʾ����NO���ڷ�Ӧ2NO(g)+2CO(g)![]() N2(g)+2CO2(g)��NO��CO�Ļ�ѧ��������ȣ�A��ʱ�����ߵ�ת������ȣ���Ϊ80%����n(CO)/n(NO)=1,��ʱ�����ʵ�Ũ�ȷֱ�Ϊ0.4mol/L��0.4mol/L��0.8mol/L��1.6mol/L����Ӧ��ƽ�ⳣ��K=
N2(g)+2CO2(g)��NO��CO�Ļ�ѧ��������ȣ�A��ʱ�����ߵ�ת������ȣ���Ϊ80%����n(CO)/n(NO)=1,��ʱ�����ʵ�Ũ�ȷֱ�Ϊ0.4mol/L��0.4mol/L��0.8mol/L��1.6mol/L����Ӧ��ƽ�ⳣ��K=![]() ��(5)��Ʒ�Ӧ2CO(g)=2C(s)+O2(g)������CO����Ⱦ�����鲻����, �����Ǹ÷�Ӧ��H>0����S<0.��H-T��S>0,�÷�Ӧ���κ�����¶������Է����С�
��(5)��Ʒ�Ӧ2CO(g)=2C(s)+O2(g)������CO����Ⱦ�����鲻����, �����Ǹ÷�Ӧ��H>0����S<0.��H-T��S>0,�÷�Ӧ���κ�����¶������Է����С�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����֪H2(g)��Br2(g)===2HBr(g)����H����72 kJ/mol����������������±���
���� | H2(g) | Br2(g) | HBr(g) |
1 mol �����еĻ�ѧ���� ��ʱ��Ҫ���յ�����/kJ | 436 | a | 369 |
�����aΪ(����)
A.404B.260C.230D.200