��Ŀ����
�������ʵ���һ���ļ����ij�л���A(����ʽΪCaHbOc��a��1��c��1��bΪ������)�Ļ���
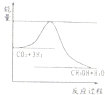
(1)��������м����A�����Ժ��ֱ�����ϣ�����ȫȼ�������ɵ�ˮ�����ʵ������䣬��A����ɱ�������������� �����ϴ�������A�У���Է���������С��A�ķ���ʽΪ ��
(2) ��������м����A�����Ժ��ֱ�����ϣ�����ȫȼ�������ĵ����������ɵ�ˮ�����ʵ��������䣬���ϴ�������A�У���Է���������С��A�ķ���ʽΪ ��
(3) ���л���CxHy(x��yΪ������)��CaHbOc(a��x)�����Ժ��ֱ�����ϣ�ֻҪ�����������ʵ���һ������ȫȼ��ʱ�����ĵ����������ɵ�ˮ�����ʵ��������䣬��ô���������л������ɱ��������������(�ú�x��y��a��b��c�ȵĴ���ʽ��ʾ)
��
��1��b=4����A����ʽ����ԭ����Ϊ4����2�֣���CH4O��2�֣�
��2�� C2H4O2��3�֣�
��3�� y=b ��2�֣� �� 2(a��x) = c��1�֣�

��ϰ��ϵ�д�
 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д� ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
�����Ŀ
��úΪԭ�ϣ�����ѧ�ӹ�ʹúת��Ϊ���塢Һ�塢����ȼ���Լ����ֻ�����Ʒ�Ĺ�ҵ��ú������
��1����ˮ����ͨ�����ȵ�̼���ɲ���ˮú������ӦΪ��C��s��+H2O��g��?CO��g��+H2��g����H=+131.3kJ?mo1-1
�ٸ÷�Ӧ�ڸ�����______�Է����У���ܡ����ܡ���
�ں����£����ݻ�������ܱ������У��������Ͽ��淴Ӧ��һ��ʱ��������������������仯ʱ���ܱ����÷�Ӧ�Ѵﵽƽ��״̬���ǣ�______��
����������ܶȣ��������������ѹǿ����������������ʵ�������CO���ʵ���Ũ��
A��ֻ�Т���
Bֻ�Т�͢�
C��ֻ�Т�͢�
D��ֻ�Т�͢�
��2��ˮú���ٽ�һ����Ӧ����ȡ��������ӦΪH2O��g��+CO��g��?H2��g��+CO2��g����ij�¶��¸÷�Ӧ��ƽ�ⳣ��K=4/9�����¶����ڼס��ҡ������������ܱ������У�ֻͶ��H2��g����CO2��g��������ʼŨ���±���ʾ�������жϲ���ȷ����______
A����Ӧ��ʼʱ�����еķ�Ӧ������죬���еķ�Ӧ����
B��ƽ��ʱ���кͱ��е�H2��ת���ʾ���60%
C��ƽ��ʱ�����е�c��CO2���Ǽ��е�2������0.102mol/L
D��ƽ��ʱ�����е�CO2��ת���ʴ���60%
��3��Ŀǰ��ҵ����һ�ַ�������CO2����ȡ�״���һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g������ͼ��ʾ�÷�Ӧ���й����������ı仯�������Ϊ1L�ĺ����ܱ������У�����1mo1CO2��3mo1H2��
�����д�ʩ����ʹƽ��������c��CH3OH�����������______
A�������¶�
B������He��g��ʹ��ϵѹǿ����
C����H2O��g������ϵ�з������
D���ٳ���1mo1CO2��3mo1H2
�����¶�T1ʱ������Ӧ�ﵽƽ��ʱ�����n��H2��=2.4mo1�������������䣬���¶�T2ʱ������Ӧ�ﵽƽ��ʱ�����n��CO2��=0.82mol�����T1______T2�����������������=����
��4����һ�������¿�ѧ�Ҵ��̵����з����CO2��̫���ܵ�ص��ˮ������H2�ϳɼ״���������ΪҺ�壩��CH3OH��H2��ȼ���ȷֱ�Ϊ����H=-725.5kJ/mo1����H=-285.5kJmo1
��д����ҵ����CO2��H2�ϳ�CH3OH��Һ̬ˮ���Ȼ�ѧ����ʽ��______
�ڸ�ת���Ļ���������______
���������������Ʒ�ӦCO2=C+O2����H��0����S��0��������CO2�Ի�����Ӱ�죮�����ж��Ƿ���в�˵�����ɣ�______��

��1����ˮ����ͨ�����ȵ�̼���ɲ���ˮú������ӦΪ��C��s��+H2O��g��?CO��g��+H2��g����H=+131.3kJ?mo1-1
�ٸ÷�Ӧ�ڸ�����______�Է����У���ܡ����ܡ���
�ں����£����ݻ�������ܱ������У��������Ͽ��淴Ӧ��һ��ʱ��������������������仯ʱ���ܱ����÷�Ӧ�Ѵﵽƽ��״̬���ǣ�______��
����������ܶȣ��������������ѹǿ����������������ʵ�������CO���ʵ���Ũ��
A��ֻ�Т���
Bֻ�Т�͢�
C��ֻ�Т�͢�
D��ֻ�Т�͢�
��2��ˮú���ٽ�һ����Ӧ����ȡ��������ӦΪH2O��g��+CO��g��?H2��g��+CO2��g����ij�¶��¸÷�Ӧ��ƽ�ⳣ��K=4/9�����¶����ڼס��ҡ������������ܱ������У�ֻͶ��H2��g����CO2��g��������ʼŨ���±���ʾ�������жϲ���ȷ����______
| ��ʼŨ�� | �� | �� | �� |
| c��H2��/mo1/L | 0.010 | 0.020 | 0.020 |
| c��CO2��/moI/L | 0.010 | 0.010 | 0.020 |
B��ƽ��ʱ���кͱ��е�H2��ת���ʾ���60%
C��ƽ��ʱ�����е�c��CO2���Ǽ��е�2������0.102mol/L
D��ƽ��ʱ�����е�CO2��ת���ʴ���60%
��3��Ŀǰ��ҵ����һ�ַ�������CO2����ȡ�״���һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g������ͼ��ʾ�÷�Ӧ���й����������ı仯�������Ϊ1L�ĺ����ܱ������У�����1mo1CO2��3mo1H2��
�����д�ʩ����ʹƽ��������c��CH3OH�����������______
A�������¶�
B������He��g��ʹ��ϵѹǿ����
C����H2O��g������ϵ�з������
D���ٳ���1mo1CO2��3mo1H2
�����¶�T1ʱ������Ӧ�ﵽƽ��ʱ�����n��H2��=2.4mo1�������������䣬���¶�T2ʱ������Ӧ�ﵽƽ��ʱ�����n��CO2��=0.82mol�����T1______T2�����������������=����
��4����һ�������¿�ѧ�Ҵ��̵����з����CO2��̫���ܵ�ص��ˮ������H2�ϳɼ״���������ΪҺ�壩��CH3OH��H2��ȼ���ȷֱ�Ϊ����H=-725.5kJ/mo1����H=-285.5kJmo1
��д����ҵ����CO2��H2�ϳ�CH3OH��Һ̬ˮ���Ȼ�ѧ����ʽ��______
�ڸ�ת���Ļ���������______
���������������Ʒ�ӦCO2=C+O2����H��0����S��0��������CO2�Ի�����Ӱ�죮�����ж��Ƿ���в�˵�����ɣ�______��

 ��ͼ���мס�����������������ɱ䣬ѹǿ���䣬�ұ���������䣮���������зֱ����1mol A��3mol B����ʱ�����������Ϊ0.5L���¶�ΪT�棬�����¶Ȳ��䷢����Ӧ��A��g��+3B��g��?xC��g��+2D��s�����������з�Ӧ��4min����n��A��=0.5mol��v��c��=0.5mol?L-1?min-1��
��ͼ���мס�����������������ɱ䣬ѹǿ���䣬�ұ���������䣮���������зֱ����1mol A��3mol B����ʱ�����������Ϊ0.5L���¶�ΪT�棬�����¶Ȳ��䷢����Ӧ��A��g��+3B��g��?xC��g��+2D��s�����������з�Ӧ��4min����n��A��=0.5mol��v��c��=0.5mol?L-1?min-1�� ��2010?������ģ��A��B��C��D����ǰ18��Ԫ����ɵ����ֳ��������DΪ����ɫ���壬�ס��������ֵ��ʣ���Щ���ʺͻ�����֮�������ͼ��Ӧ��ϵ��
��2010?������ģ��A��B��C��D����ǰ18��Ԫ����ɵ����ֳ��������DΪ����ɫ���壬�ס��������ֵ��ʣ���Щ���ʺͻ�����֮�������ͼ��Ӧ��ϵ��