��Ŀ����
����Ŀ�����������п( ![]() )��һ�ֱ�����Լ����ܹ������ץ��������ĥ�ԡ���ش��������⣺
)��һ�ֱ�����Լ����ܹ������ץ��������ĥ�ԡ���ش��������⣺
(1)���������п�ṹ�У�P���ӻ���ʽΪ__________________��C1��P��S�ĵ�һ�������ɴ�С��˳��Ϊ_____________________��
(2)Zn2+�ļ۲�����Ų�ʽΪ________________��п�ܹ���ǿ����Һ��Ӧ����[Zn(OH)4]2-�������ǿռ乹�ͣ�[Zn(OH)4]2���Ľṹ����ʾ��ͼ��ʾΪ__________________��
(3)��Ԫ�����γɶ��ֺ����ᣬ������������ȥһ����ˮ���ɽ�����(H2S2O7)����1mol�������к�������������ĿΪ____________���ü۲���ӻ��������жϲ�����SO32-��SO42-���ǵ���Դ�С��
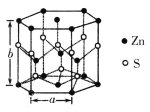
(4)��п�����������ṹ��ͼ��ʾ���þ����Ļ�ѧʽΪ___________���侧�������ֱ�Ϊa pm��b pm����þ�����ܶ�Ϊ____________________g��cm-3(�ú�a��b��NA�Ĵ���ʽ��ʾ)��
���𰸡�sp3 Cl>P>S 3d10 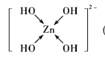 8NA ZnS
8NA ZnS ![]()
��������
(1)���ݶ��������п�ṹ�ж��ӻ���ʽ��ͬ�����У���һ��������ԭ�����������������P���������Ӵ��ڰ����״̬�����ȶ���
(2) Znʧȥ�������Ӻ��γ�Zn2+��[Zn(OH)4]2-�У�Zn2+��OH-�γ�4����λ����
(3)������������ȥһ����ˮ���ɽ�����(H2S2O7)ʱ������������Ŀδ�䣻
(4)������п����Ľṹ��Zn�ڶ��㡢���ĺ��ڲ���S�����Ϻ��ڲ��������ܶ�=![]() ���м��㡣
���м��㡣
(1)���ݶ��������п�ṹ��P����4���������µ��Ӷԣ����ӻ���ʽΪsp3��ͬ�����У���һ��������ԭ�����������������P�ļ۲�����Ų�ʽΪ3s23p3�����ڰ����״̬�����ȶ��������һ�����ܸ���S�ģ���һ�������ɴ�С��˳��ΪCl>P>S��
(2) Zn�ļ۲�����Ų�ʽΪ3d104s2����ʧȥ�������Ӻ��γ�Zn2+����۲�����Ų�ʽΪ3d10��[Zn(OH)4]2-�У�Zn2+��OH-�γ�4����λ������ṹʾ��ͼΪ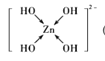 ��
��
(3)��������У�Sԭ������4��Oԭ�ӣ��γ�4����������������������ȥһ����ˮ���ɽ�����(H2S2O7)ʱ��H-O���Ͽ����Oԭ����������һ����������е�Sԭ�ӣ�����������Ŀδ�䣬��1mol�������к�������������ĿΪ8NA��
(4)������п����Ľṹ��Zn�ڶ��㡢���ĺ��ڲ�������=2��![]() +12��
+12��![]() +3=6��S�����Ϻ��ڲ�������=6��
+3=6��S�����Ϻ��ڲ�������=6��![]() +4=6����ѧʽΪZnS���þ����ĵ����Ϊ�������Σ������=
+4=6����ѧʽΪZnS���þ����ĵ����Ϊ�������Σ������=![]() pm3��1mol����������=��65+32����6=582g��������ܶ�=
pm3��1mol����������=��65+32����6=582g��������ܶ�=![]() =
=![]() =
=![]() g/cm3��
g/cm3��

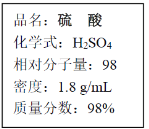
����Ŀ���ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�����塣 �ϳ����з�����ӦΪ�� N2(g)+3H2(g)![]() 2NH3(g) ��H<0
2NH3(g) ��H<0
(1)��ҵ����ʱ����ȡ������һ����ӦΪ��CO(g)+H2O(g)![]() CO2(g)+H2(g) ��H>0
CO2(g)+H2(g) ��H>0
��t��ʱ����1L�ܱ������г���0.2mol CO��0.3molˮ��������Ӧ����ƽ�����ϵ��c(H2)��0.12mol��L��1��
���¶��´˷�Ӧ��ƽ�ⳣ��K ��__________________________��
�������¶Ȳ��䣬������ƽ����ϵ���ټ���0.1mol CO������Ӧ���½���ƽ��ʱ��ˮ��������ת������ (H2O)__________��
(2)�±�Ϊ��ͬ�¶��ºϳɰ���Ӧ��ƽ�ⳣ�����ɱ�����֪T1_____573K(����>������<������=��)��
T/K | T1 | 573 | T2 |
K | 1.00��107 | 2.45��105 | 1.88��103 |
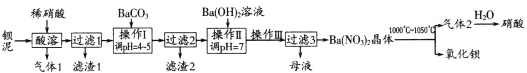
(3)673K��30MPa��n(NH3) ��n(H2) ��ʱ��仯�Ĺ�ϵ����ͼ��ʾ������ͼ�������й�������ȷ����(��д���)_________________��

A��c���ʾn(NH3)��n (H2)���
B��c���ʾNH3����������NH3�ֽ�������ͬ
C��e���d��ʱ��Ӧ��ƽ�ⳣ����ͬ
D��c��ʱ����Ӧ���ʴ����淴Ӧ����
(4)��NO2����ˮ�����3NO2+H2O![]() 2HNO3+NO�����������̿�������������
2HNO3+NO�����������̿�������������
Ҫ�����������ԭ���û�ѧƽ���ƶ������۽��н�����______________��
(5)���᳧��β�����е��������������ֱ���ŷŽ���Ⱦ������Ŀǰ��ѧ��̽��
����ȼ�������еļ���Ƚ��������ﻹԭΪ������ˮ���䷴Ӧ����Ϊ��
CH4(g)+4NO2(g)��4NO(g)+CO2(g)+2H2O(g)�� ��H����574kJ��mol��1
CH4(g)+4NO(g)��2N2(g)+CO2(g)+2H2O(g)�� ��H����1160kJ��mol��1
�����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ��_______________________��
����Ŀ���±�ΪԪ�����ڱ���һ���֡�
̼ | �� | Y | |
X | �� | Z |
�ش��������⣺
��1��ZԪ�������ڱ��е�λ��Ϊ___��
��2��������ʵ��˵��YԪ�صķǽ����Ա�SԪ�صķǽ�����ǿ����___��
a��Y������H2S��Һ��Ӧ����Һ�����
b����������ԭ��Ӧ�У�1molY���ʱ�1molS�õ��Ӷ�
c��Y��S��Ԫ�صļ��⻯�����ȷֽ⣬ǰ�ߵķֽ��¶ȸ�
��3��X��Z��Ԫ�صĵ��ʷ�Ӧ����1molX����ۻ�����ָ������£�����687kJ����֪�û�������ۡ��е�ֱ�Ϊ-69���58�棬д���÷�Ӧ���Ȼ�ѧ����ʽ___��
��4��̼��þ�γɵ�1mol������Q��ˮ��Ӧ������2molMg(OH)2��1mol��������������̼��������Ϊ9��1�����ĵ���ʽΪ___��Q��ˮ��Ӧ�Ļ�ѧ����ʽΪ__��