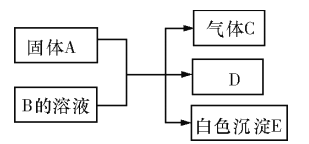
��Ŀ����
����Ŀ����ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��г��豸���ԣ���

��1���Ʊ�����ѡ�õ�ҩƷΪ��Ư�۾������Ũ���ᣬ��صĻ�ѧ��Ӧ����ʽΪ��
___________________________________________________________��
��2��װ��B�б���ʳ��ˮ��������_______________��ͬʱװ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е�����___________________��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C��I��II��III���η���_______��
a | b | c | d | |
I | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
II | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
III | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��4�����װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ�����D�л���ͨ��һ��������ʱ�����Կ�����ɫ��Һ��Ϊ______ɫ��˵���ȵķǽ����Դ����塣
��5����������װ��D��������Һ����װ��E�У����۲쵽��������_____________��
��6�����������װ��F�пɸ���������NaHSO3��Һ�������ȣ���д����Ӧ�����ӷ�Ӧ����ʽ��__________________���жϸ���NaHSO3��Һ�Ƿ����______����ǡ�����
���𰸡� Ca(ClO)2 + 4HCl��Ũ��=== CaCl2 + 2Cl2��+ 2H2O ��ȥCl2�е�HCl B�г���©����Һ���������γ�ˮ�� d �� E����Һ��Ϊ���㣬�ϲ㣨���㣩Ϊ�Ϻ�ɫ HSO3�� + Cl2 + H2O === SO42�� + 2Cl�� + 3H+����4HSO3�� + Cl2 �� SO42�� + 2Cl�� + 3SO2 + 2H2O�� ��
����������1��Ư�۾�����Ҫ�ɷ��Ǵ������ƣ��������ƾ��������ԣ���Ũ���ᷴӦ����������ԭ��Ӧ��������������ʽΪCa(ClO)2 + 4HCl��Ũ����CaCl2 + 2Cl2��+ 2H2O����2��Ũ������лӷ��ԣ�װ��B�б���ʳ��ˮ�����������ջӷ��������Ȼ������壻���C�з���������װ���������ѹǿ�ͻ�����B�ij���©����Һ��ͻ��������γ�ˮ������3�����������û��Ư���ԣ�ʪ���������Ư���ԣ�����װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ����ڴӱ���ʳ��ˮ�����������к���ˮ������I��ֻ����ʪ�����ɫ������II����������Ӧѡ�����������ˮ�Ȼ��ƣ�III����֤����������Ƿ���Ư���ԣ���ѡd����4������D�л���ͨ��һ��������ʱ���������û����壬��ˮ�ʳȻ�ɫ������ɫ��Һ��Ϊ��ɫʱ��˵���ȵķǽ����Դ����塣��5��������װ��D�е���Һ�к����壬�������װ��E�У����û����⣬���ܹ���ȡ��ˮ�еĵ�����Ϻ�ɫ�����۲쵽��������E����Һ��Ϊ���㣬�ϲ㣨���㣩Ϊ�Ϻ�ɫ����6��NaHSO3��Һ���л�ԭ�ԣ��������������ԣ�����������ԭ��Ӧ�����������ƣ�������������ԭ�������ӣ���Ӧ����Һ�����ԣ����������NaHSO3��Һ��Ӧ�ų�SO2����Ⱦ��������˲�����NaHSO3��Һ�������ȣ���Ӧ�ķ���ʽΪ4HSO3�� + Cl2 ��SO42�� + 2Cl�� + 3SO2 + 2H2O��

����Ŀ����һ��������ܱ������У��������»�ѧ��Ӧ��CO2(g) +H2(g)![]() CO (g) +H2O (g)
CO (g) +H2O (g)
�仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
t�� | 700 | 800 | 830 | 1000 | 1200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�ش��������⣺
��1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK��__________��
��2����˵���÷�Ӧ�ﵽ��ѧƽ��״̬����________��
A��������ѹǿ���� B�����������c(CO)����
C��v��(H2)��v��(H2O) D��c(CO2)��c(CO)
��3��ij�¶��£�ƽ��Ũ�ȷ�����ʽ�� 3c(CO2)��c(H2)��5c(CO)��c(H2O)�����жϴ�ʱ���¶�Ϊ______�档
��4�� 830��ʱ����1L�ܱ������зֱ�Ͷ��lmolH2��lmolCO2��Ӧ�ﵽ��ѧƽ��ʱ��CO2��ת����Ϊ__________�������¶Ȳ��䣬��ƽ����ϵ���ٳ���1molH2��lmolCO2���´ﵽ��ѧƽ��ʱ��CO2��ƽ��ת����_________(���������С�����䡱)��