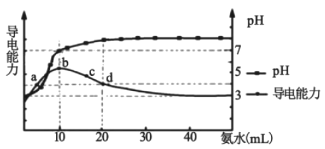
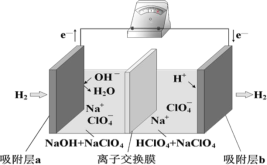
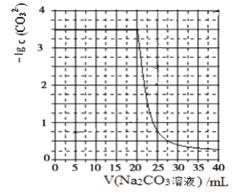
��Ŀ����
����Ŀ��ij�����о���ѧϰС������ͼ��ʾװ���Ʊ������屽����֤���뱽�ķ�Ӧ��ȡ����Ӧ��
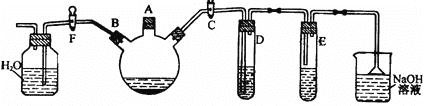
ʵ��ʱ���ر�F��������C��������װ����������������ƿ����A�ڼ�������Һ�壬�ټ���������м����סA�ڡ��ش��������⣺
��1��������ƿ�з����Ļ�ѧ��Ӧ����ʽΪ_______��
��2��D�Թ���װ����CCl4��Һ����������_______��
��3����ȥ�屽�л��е�Br2���ʵ��Լ���______����������Ϊ:___���йػ�ѧ����ʽ________��
��4����Ӧ��Ϻ��Թ�E(AgNO3��Һ)����___�����������ɣ�˵�����ֻ���屽�ķ�Ӧ����_______��Ӧ��
���𰸡�![]() +Br2
+Br2 +HBr ���ջӷ�������Br2���� NaOH ������ƿ�м�������NaOH��Һ����ת���Һ©������Һ Br2+ 2NaOH=NaBr + NaBrO + H2O ����ɫ���� ȡ��
+HBr ���ջӷ�������Br2���� NaOH ������ƿ�м�������NaOH��Һ����ת���Һ©������Һ Br2+ 2NaOH=NaBr + NaBrO + H2O ����ɫ���� ȡ��
��������
��1��������ƿ�еķ�Ӧ��Ϊ�������������м�������巢��ȡ����Ӧ��
��2��������ķ�ӦΪ���ȷ�Ӧ������������ӷ���D�Թ���װ����CCl4��Һ�������ջӷ�������������
��3���屽�л��е�Br2����Ϊ��ܽ⣬�����NaOH��Ӧ���ɿ�����ˮ���ζ����屽�з��룬��������ƿ�м�������NaOH��Һ����ת���Һ©������Һ��
��4����Ӧ�в�����HBr�����Թ�E�У��������������ӷ�Ӧ���ɵ���ɫ����AgBr��˵�����뱽����ȡ����Ӧ��
��1��������ƿ�еķ�Ӧ��Ϊ�������������м�������巢��ȡ����Ӧ����ѧ��Ӧ����ʽΪ![]() +Br2
+Br2 +HBr��
+HBr��
��2��������ķ�ӦΪ���ȷ�Ӧ������������ӷ���D�Թ���װ����CCl4��Һ�������ջӷ�������������
��3���屽�л��е�Br2����Ϊ��ܽ⣬�����NaOH��Ӧ���ɿ�����ˮ���ζ����屽�з��룬��������ƿ�м�������NaOH��Һ����ת���Һ©������Һ����Ӧ�ķ���ʽΪ��Br2 + 2NaOH= NaBr + NaBrO + H2O��
��4����Ӧ�в�����HBr�����Թ�E�У��������������ӷ�Ӧ���ɵ���ɫ����AgBr��˵�����뱽����ȡ����Ӧ��