ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΚœ≥…“Ϋ”Ο¬ιΉμ“©ή–ΉτΩ®“ρEΚΆ ≥ΤΖΖάΗ·ΦΝJΒΡ¬ΖœΏ»γΆΦΥυ ΨΘΚ
“―÷ΣΘΚ
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©A τ”ΎΖΦœψΧΰΘ§ΫαΙΙΦρ ΫΈΣ_____________ΓΘ
Θ®2Θ©E÷–ΙΌΡήΆ≈ΒΡΟϊ≥Τ «Α±ΜυΓΔ____________ΓΘ
Θ®3Θ©CΡή”κNaHCO3»ή“ΚΖ¥”ΠΘ§Ζ¥”ΠΔέΒΡΜ·―ßΖΫ≥Χ Ϋ «___________ΓΘ
Θ®4Θ©Ζ¥”ΠΔόΓΔΔΏ÷– ‘ΦΝiiΚΆ ‘ΦΝiii“ά¥Έ « ___________ΓΔ___________ΓΘ
Θ®5Θ©Ζ¥”ΠΔΌ~ΔΏ÷–Θ§ τ”Ύ»Γ¥ζΖ¥”ΠΒΡ «_______________ΓΘ
Θ®6Θ©J”–Εύ÷÷Ά§Ζ÷“λΙΙΧεΘ§Τδ÷–ΖϊΚœœ¬Ν–ΧθΦΰΒΡΆ§Ζ÷“λΙΙΧε”–______________÷÷Θ§–¥≥ωΤδ÷–»Έ“Μ÷÷Ά§Ζ÷“λΙΙΧεΒΡΫαΙΙΦρ ΫΘΚ___________ΓΘ
a. ΈΣ±ΫΒΡΕΰ‘Σ»Γ¥ζΈοΘ§Τδ÷–“ΜΗω»Γ¥ζΜυΈΣτ«Μυ
b. τ”ΎθΞάύΘ§«“ΡήΖΔ…ζ“χΨΒΖ¥”Π
Θ®7Θ©“‘AΈΣΤπ Φ‘≠ΝœΘ§―ÔϱΊ“ΣΒΡΈόΜζ ‘ΦΝΚœ≥…ΗΏΖ÷Ή” ς÷§Θ®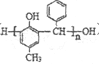 Θ©Θ§–¥≥ωΚœ≥…¬ΖœΏΘ®”ΟΫαΙΙΦρ Ϋ±μ Ψ”–ΜζΈοΘ§”ΟΦΐΆΖ±μ ΨΉΣΜ·ΙΊœΒΘ§ΦΐΆΖ…œΉΔΟς ‘ΦΝΚΆΖ¥”ΠΧθΦΰΘ©ΘΚ_______________ΓΘ
Θ©Θ§–¥≥ωΚœ≥…¬ΖœΏΘ®”ΟΫαΙΙΦρ Ϋ±μ Ψ”–ΜζΈοΘ§”ΟΦΐΆΖ±μ ΨΉΣΜ·ΙΊœΒΘ§ΦΐΆΖ…œΉΔΟς ‘ΦΝΚΆΖ¥”ΠΧθΦΰΘ©ΘΚ_______________ΓΘ
ΓΨ¥πΑΗΓΩ ![]() θΞΜυΘ®ΦϋΘ©
θΞΜυΘ®ΦϋΘ© ![]() Υα–‘ΗΏΟΧΥαΦΊ»ή“Κ NaOH/H2O ΔΌΔέΔίΔΏ 6 Θ®“‘œ¬ΫαΙΙ»Έ–¥“Μ÷÷Θ©
Υα–‘ΗΏΟΧΥαΦΊ»ή“Κ NaOH/H2O ΔΌΔέΔίΔΏ 6 Θ®“‘œ¬ΫαΙΙ»Έ–¥“Μ÷÷Θ©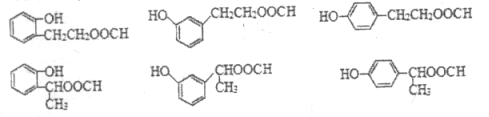

Θ®¬ΖœΏΚœάμΦ¥Ω…Θ©
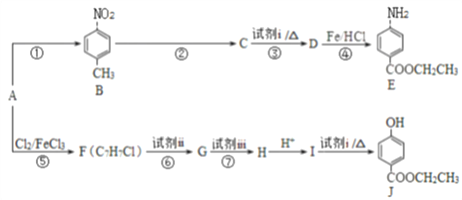
ΓΨΫβΈωΓΩ ‘ΧβΖ÷ΈωΘΚ±ΨΧβΩΦ≤ι”–ΜζΆΤΕœΚΆ”–ΜζΚœ≥…Θ§…φΦΑ”–ΜζΈοΙΌΡήΆ≈ΒΡ Ε±πΘ§”–ΜζΈοΫαΙΙΦρ ΫΚΆ”–ΜζΖ¥”ΠΖΫ≥Χ ΫΒΡ ι–¥Θ§”–ΜζΖ¥”Πάύ–ΆΒΡ≈–ΕœΘ§œόΕ®ΧθΦΰœ¬Ά§Ζ÷“λΙΙΧε ΐΡΩΒΡ»ΖΕ®ΚΆ ι–¥Θ§”–ΜζΚœ≥…¬ΖœΏΒΡ…ηΦΤΓΘA τ”ΎΖΦœψΧΰΘ§AΖΔ…ζΖ¥”ΠΔΌ…ζ≥…BΘ§AΒΡΫαΙΙΦρ ΫΈΣ![]() ΘΜΕ‘±»BΚΆEΒΡΫαΙΙΦρ Ϋ“‘ΦΑΖ¥”ΠΔήΒΡΧθΦΰΘ§Ζ¥”ΠΔή «ΫΪ-NO2ΜΙ‘≠ΈΣ-NH2Θ§DΒΡΫαΙΙΦρ ΫΈΣ
ΘΜΕ‘±»BΚΆEΒΡΫαΙΙΦρ Ϋ“‘ΦΑΖ¥”ΠΔήΒΡΧθΦΰΘ§Ζ¥”ΠΔή «ΫΪ-NO2ΜΙ‘≠ΈΣ-NH2Θ§DΒΡΫαΙΙΦρ ΫΈΣ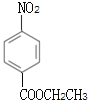 ΘΜCΡή”κNaHCO3»ή“ΚΖ¥”ΠΘ§C÷–Κ§τ»ΜυΘ§Ζ¥”ΠΔΎΈΣ”ΟΥα–‘ΗΏΟΧΥαΦΊ»ή“ΚΫΪB÷–-CH3―θΜ·≥…-COOHΘ§CΒΡΫαΙΙΦρ ΫΈΣ
ΘΜCΡή”κNaHCO3»ή“ΚΖ¥”ΠΘ§C÷–Κ§τ»ΜυΘ§Ζ¥”ΠΔΎΈΣ”ΟΥα–‘ΗΏΟΧΥαΦΊ»ή“ΚΫΪB÷–-CH3―θΜ·≥…-COOHΘ§CΒΡΫαΙΙΦρ ΫΈΣ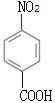 ΘΜΖ¥”ΠΔέΈΣC”κCH3CH2OHΒΡθΞΜ·Ζ¥”ΠΘ§ ‘ΦΝiΈΣCH3CH2OHΓΔ≈®ΝρΥαΓΘΫαΚœFΒΡΖ÷Ή” ΫΘ§Ζ¥”ΠΔίΈΣA”κCl2‘Ύ¥ΏΜ·ΦΝ¥φ‘Ύœ¬ΖΔ…ζ±ΫΜΖ…œΒΡ»Γ¥ζΖ¥”ΠΘ§”…”ΎJ÷–ΝΫΗω≤ύΝ¥¥Π”ΎΕ‘ΈΜΘ§‘ρFΒΡΫαΙΙΦρ ΫΈΣ
ΘΜΖ¥”ΠΔέΈΣC”κCH3CH2OHΒΡθΞΜ·Ζ¥”ΠΘ§ ‘ΦΝiΈΣCH3CH2OHΓΔ≈®ΝρΥαΓΘΫαΚœFΒΡΖ÷Ή” ΫΘ§Ζ¥”ΠΔίΈΣA”κCl2‘Ύ¥ΏΜ·ΦΝ¥φ‘Ύœ¬ΖΔ…ζ±ΫΜΖ…œΒΡ»Γ¥ζΖ¥”ΠΘ§”…”ΎJ÷–ΝΫΗω≤ύΝ¥¥Π”ΎΕ‘ΈΜΘ§‘ρFΒΡΫαΙΙΦρ ΫΈΣ![]() ΘΜ ”…”Ύ ‘ΦΝiΈΣCH3CH2OHΓΔ≈®ΝρΥαΘ§”…JΡφΆΤ≥ωIΒΡΫαΙΙΦρ ΫΈΣ
ΘΜ ”…”Ύ ‘ΦΝiΈΣCH3CH2OHΓΔ≈®ΝρΥαΘ§”…JΡφΆΤ≥ωIΒΡΫαΙΙΦρ ΫΈΣ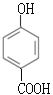 ΘΜΕ‘±»FΚΆIΒΡΫαΙΙΦρ ΫΘ§FΉΣΜ·ΈΣI–η“ΣΫΪ-CH3―θΜ·≥…-COOHΘ§ΫΪ-ClΥ°Ϋβ≥…Ζ”τ«ΜυΘ§”…”ΎΖ”τ«Μυ“≤“ΉΖΔ…ζ―θΜ·Ζ¥”ΠΘ§ΈΣΝΥ±ΘΜΛΖ”τ«Μυ≤Μ±Μ―θΜ·Θ§Ζ¥”ΠΔόΈΣ―θΜ·Ζ¥”ΠΘ§Ζ¥”ΠΔΏΈΣΥ°ΫβΖ¥”ΠΘ§‘ρ ‘ΦΝiiΈΣΥα–‘KMnO4»ή“ΚΘ§GΒΡΫαΙΙΦρ ΫΈΣ
ΘΜΕ‘±»FΚΆIΒΡΫαΙΙΦρ ΫΘ§FΉΣΜ·ΈΣI–η“ΣΫΪ-CH3―θΜ·≥…-COOHΘ§ΫΪ-ClΥ°Ϋβ≥…Ζ”τ«ΜυΘ§”…”ΎΖ”τ«Μυ“≤“ΉΖΔ…ζ―θΜ·Ζ¥”ΠΘ§ΈΣΝΥ±ΘΜΛΖ”τ«Μυ≤Μ±Μ―θΜ·Θ§Ζ¥”ΠΔόΈΣ―θΜ·Ζ¥”ΠΘ§Ζ¥”ΠΔΏΈΣΥ°ΫβΖ¥”ΠΘ§‘ρ ‘ΦΝiiΈΣΥα–‘KMnO4»ή“ΚΘ§GΒΡΫαΙΙΦρ ΫΈΣ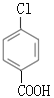 ΘΜ ‘ΦΝiiiΈΣNaOH»ή“ΚΘ§HΒΡΫαΙΙΦρ ΫΈΣ
ΘΜ ‘ΦΝiiiΈΣNaOH»ή“ΚΘ§HΒΡΫαΙΙΦρ ΫΈΣ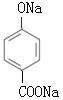 ΓΘ
ΓΘ
Θ®1Θ©A τ”ΎΖΦœψΧΰΘ§AΒΡΫαΙΙΦρ ΫΈΣ![]() ΓΘ
ΓΘ
Θ®2Θ©”…EΒΡΫαΙΙΦρ ΫΩ…÷ΣΘ§E÷–ΙΌΡήΆ≈ΒΡΟϊ≥ΤΈΣΑ±ΜυΓΔθΞΜυΓΘ
Θ®3Θ©CΒΡΫαΙΙΦρ ΫΈΣ Θ§Ζ¥”ΠΔέΈΣC”κCH3CH2OHΒΡθΞΜ·Ζ¥”ΠΘ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ
Θ§Ζ¥”ΠΔέΈΣC”κCH3CH2OHΒΡθΞΜ·Ζ¥”ΠΘ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ +CH3CH2OH
+CH3CH2OH![]()
 +H2OΓΘ
+H2OΓΘ
Θ®4Θ©Ζ¥”ΠΔό «ΫΪ![]() ÷–-CH3―θΜ·≥…-COOHΘ§ ‘ΦΝii «Υα–‘ΗΏΟΧΥαΦΊ»ή“ΚΓΘΖ¥”ΠΔΏ «
÷–-CH3―θΜ·≥…-COOHΘ§ ‘ΦΝii «Υα–‘ΗΏΟΧΥαΦΊ»ή“ΚΓΘΖ¥”ΠΔΏ « ΒΡΥ°ΫβΖ¥”ΠΘ§ ‘ΦΝiii «NaOH»ή“ΚΓΘ
ΒΡΥ°ΫβΖ¥”ΠΘ§ ‘ΦΝiii «NaOH»ή“ΚΓΘ
Θ®5Θ©Ζ¥”ΠΔΌ~ΔΏ“ά¥ΈΈΣ»Γ¥ζΖ¥”ΠΓΔ―θΜ·Ζ¥”ΠΓΔ»Γ¥ζΖ¥”ΠΓΔΜΙ‘≠Ζ¥”ΠΓΔ»Γ¥ζΖ¥”ΠΓΔ―θΜ·Ζ¥”ΠΓΔ»Γ¥ζΖ¥”ΠΘ§ τ”Ύ»Γ¥ζΖ¥”ΠΒΡ «ΔΌΔέΔίΔΏΓΘ
Θ®6Θ©JΒΡΫαΙΙΦρ ΫΈΣ Θ§JΒΡΆ§Ζ÷“λΙΙΧε τ”ΎθΞάύ«“ΡήΖΔ…ζ“χΨΒΖ¥”ΠΘ§‘ρΫαΙΙ÷–Κ§”–HCOO-Θ§JΒΡΆ§Ζ÷“λΙΙΧεΈΣ±ΫΒΡΕΰ‘Σ»Γ¥ζΈοΘ§Τδ÷–“ΜΗω»Γ¥ζΜυΈΣ-OHΘΜΖϊΚœΧθΦΰΒΡΆ§Ζ÷“λΙΙΧε”–ΘΚΘ®1Θ©»τΝΫΗω»Γ¥ζΜυΈΣ-OHΚΆ-CH2CH2OOCHΘ§”–ΝΎΓΔΦδΓΔΕ‘»ΐΗωΈΜ÷ΟΘ§ΫαΙΙΦρ ΫΈΣ
Θ§JΒΡΆ§Ζ÷“λΙΙΧε τ”ΎθΞάύ«“ΡήΖΔ…ζ“χΨΒΖ¥”ΠΘ§‘ρΫαΙΙ÷–Κ§”–HCOO-Θ§JΒΡΆ§Ζ÷“λΙΙΧεΈΣ±ΫΒΡΕΰ‘Σ»Γ¥ζΈοΘ§Τδ÷–“ΜΗω»Γ¥ζΜυΈΣ-OHΘΜΖϊΚœΧθΦΰΒΡΆ§Ζ÷“λΙΙΧε”–ΘΚΘ®1Θ©»τΝΫΗω»Γ¥ζΜυΈΣ-OHΚΆ-CH2CH2OOCHΘ§”–ΝΎΓΔΦδΓΔΕ‘»ΐΗωΈΜ÷ΟΘ§ΫαΙΙΦρ ΫΈΣ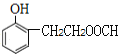 ΓΔ
ΓΔ ΓΔ
ΓΔ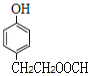 ΘΜΘ®2Θ©»τΝΫΗω»Γ¥ζΜυΈΣ-OHΚΆ-CHΘ®CH3Θ©OOCHΘ§”–ΝΎΓΔΦδΓΔΕ‘»ΐΗωΈΜ÷ΟΘ§ΫαΙΙΦρ ΫΈΣ
ΘΜΘ®2Θ©»τΝΫΗω»Γ¥ζΜυΈΣ-OHΚΆ-CHΘ®CH3Θ©OOCHΘ§”–ΝΎΓΔΦδΓΔΕ‘»ΐΗωΈΜ÷ΟΘ§ΫαΙΙΦρ ΫΈΣ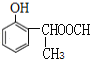 ΓΔ
ΓΔ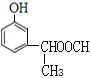 ΓΔ
ΓΔ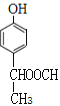 ΘΜΖϊΚœΧθΦΰΒΡΆ§Ζ÷“λΙΙΧεΙ≤6÷÷ΓΘ
ΘΜΖϊΚœΧθΦΰΒΡΆ§Ζ÷“λΙΙΧεΙ≤6÷÷ΓΘ
Θ®7Θ©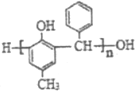 ”…ΒΞΧε
”…ΒΞΧε![]() ΚΆ
ΚΆ![]() ΖΔ…ζΥθΨέΖ¥”Π÷ΤΒΟΘ§Κœ≥…
ΖΔ…ζΥθΨέΖ¥”Π÷ΤΒΟΘ§Κœ≥… –η“Σ”…
–η“Σ”…![]() Κœ≥…
Κœ≥…![]() ΚΆ
ΚΆ![]() ΘΜ”…
ΘΜ”…![]() Κœ≥…
Κœ≥…![]() Θ§–η“Σ‘Ύ-CH3ΒΡΕ‘ΈΜ“ΐ»κ-OHΘ§œ»”…
Θ§–η“Σ‘Ύ-CH3ΒΡΕ‘ΈΜ“ΐ»κ-OHΘ§œ»”…![]() ”κCl2‘Ύ¥ΏΜ·ΦΝ¥φ‘Ύœ¬ΖΔ…ζ±ΫΜΖ…œΒΡ»Γ¥ζΖ¥”Π…ζ≥…
”κCl2‘Ύ¥ΏΜ·ΦΝ¥φ‘Ύœ¬ΖΔ…ζ±ΫΜΖ…œΒΡ»Γ¥ζΖ¥”Π…ζ≥…![]() Θ§»ΜΚσ
Θ§»ΜΚσ![]() ‘ΎNaOH»ή“Κ÷–ΖΔ…ζΥ°ΫβΖ¥”Π…ζ≥…
‘ΎNaOH»ή“Κ÷–ΖΔ…ζΥ°ΫβΖ¥”Π…ζ≥…![]() Θ§
Θ§![]() ΥαΜ·ΒΟ
ΥαΜ·ΒΟ![]() ΘΜ”…
ΘΜ”…![]() Κœ≥…
Κœ≥…![]() Θ§‘Ύ≤ύΝ¥…œ“ΐ»κΙΌΡήΆ≈Θ§œ»”…
Θ§‘Ύ≤ύΝ¥…œ“ΐ»κΙΌΡήΆ≈Θ§œ»”…![]() ”κCl2‘ΎΙβ’’œ¬ΖΔ…ζ≤ύΝ¥…œΒΡ»Γ¥ζΖ¥”Π…ζ≥…
”κCl2‘ΎΙβ’’œ¬ΖΔ…ζ≤ύΝ¥…œΒΡ»Γ¥ζΖ¥”Π…ζ≥…![]() Θ§
Θ§![]() ‘ΎNaOH»ή“Κ÷–ΖΔ…ζΥ°ΫβΖ¥”Π…ζ≥…
‘ΎNaOH»ή“Κ÷–ΖΔ…ζΥ°ΫβΖ¥”Π…ζ≥…![]() Θ§
Θ§![]() ΖΔ…ζ¥ΏΜ·―θΜ·…ζ≥…
ΖΔ…ζ¥ΏΜ·―θΜ·…ζ≥…![]() ΓΘΚœ≥…¬ΖœΏΈΣΘΚ
ΓΘΚœ≥…¬ΖœΏΈΣΘΚ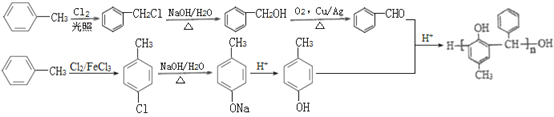 ΓΘ
ΓΘ