��Ŀ����
����Ŀ��ij��ԭ�ӵ�������ag��һ��12Cԭ�ӵ�������bg����NAֻ��ʾ�����ӵ����̵���ֵ��������˵������ȷ���ǣ���
������ԭ�ӵ����ԭ������Ϊ12a/b
��m g����ԭ�ӵ����ʵ���Ϊm/aNA mol
������ԭ�ӵ�Ħ��������aNAg/mol
�� ag����ԭ�������ĵ�����Ϊ16
��cg ����ԭ������������Ϊ4bcNA/3a
A���٢ڢۢܢ� B���٢ڢۢ� C���٢ڢܢ� D���٢ۢܢ�
���𰸡�A
��������
���������������ԭ�ӵ����ԭ������=![]() ��
��![]() ����ȷ����1mol̼ԭ�ӵĸ�����NA��n=
����ȷ����1mol̼ԭ�ӵĸ�����NA��n=![]() =
=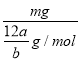 ��
��![]() mol����ȷ����Ħ����������ֵ�ϵ��������ԭ��������Ҳ����1molԭ�Ӻ��е����������Ը���ԭ�ӵ�Ħ��������aNA g/mol����ȷ����a g����ԭ�ӵĸ���=
mol����ȷ����Ħ����������ֵ�ϵ��������ԭ��������Ҳ����1molԭ�Ӻ��е����������Ը���ԭ�ӵ�Ħ��������aNA g/mol����ȷ����a g����ԭ�ӵĸ���=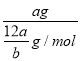 NA��
NA��![]() NA��һ����ԭ���к���16�����ӣ�����a g����ԭ�������ĵ�����Ϊ
NA��һ����ԭ���к���16�����ӣ�����a g����ԭ�������ĵ�����Ϊ![]() NA���������ڸ���ԭ�ӵ�������a g����ag����ԭ����ĿΪ1�����ʺ��е�����ĿΪ16����ȷ����cg ����ԭ������������Ϊ
NA���������ڸ���ԭ�ӵ�������a g����ag����ԭ����ĿΪ1�����ʺ��е�����ĿΪ16����ȷ����cg ����ԭ������������Ϊ![]() ����ȷ����ѡA��
����ȷ����ѡA��

��ϰ��ϵ�д�
�����Ŀ