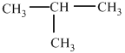
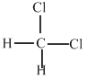
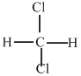
��Ŀ����
����Ŀ��ijͬѧ���÷���м(��Ҫ�ɷ�ΪFe2O3��Fe������̼)��ȡ̼��������FeCO3�����������ͼ���̣���������ͼ������˵������ȷ����
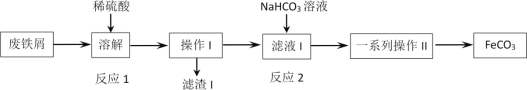
A.��ҵ����м������������֬����ͨ���ȱ���̼������Һϴ�ӳ�ȥ
B.��Ӧ2�����ӷ���ʽ��Fe2++HCO3-=FeCO3��+H+
C.����IΪ���ˣ�ϴ�Ӳ�����һϵ�в���IIΪ����Ũ������ȴ�ᾧ�����ˣ�ϴ��
D.Ϊ���������ܽ�ʱFe2+����������������мӦ�ʵ�����
���𰸡�BC
��������
����м(��Ҫ�ɷ�ΪFe2O3��Fe������̼)�м���ϡ���ᣬ������ӦFe2O3+3H2SO4=Fe2(SO4)3+3H2O��Fe2(SO4)3+Fe=3FeSO4��Fe+H2SO4=FeSO4+H2�������ˣ�������̼�˳�����Һ�м���NaHCO3��Һ��������ӦFe2++2HCO3- =FeCO3��+CO2��+H2O�����˺�ϴ�ӡ��������FeCO3��
A��ϴ�ӷ���м�������֬����ʹ���ȵı���̼������Һ��A��ȷ��
B�������Ϸ���֪����Ӧ2�����ӷ���ʽ��Fe2++2HCO3- =FeCO3��+CO2��+H2O��B����ȷ��
C����Ϊ����II�Ǵӻ�����з����FeCO3����������ӦΪ���ˡ�ϴ�ӡ�����Ȳ�����C����ȷ��
D��Fe2+���н�ǿ�Ļ�ԭ�ԣ�ϴ��ʱ�ױ������е��������������Է���мӦ�ʵ�������D��ȷ��
��ѡBC��

����Ŀ��TiCl4�����Ѿ�����Ҫ�ɷ�ΪTiO2���Ʊ��ѣ�Ti������Ҫ�м����Ʊ���TiCl4������ʾ��ͼ���£�
![]()
���ϣ�TiCl4�����������Ȼ��������
������ | SiCl4 | TiCl4 | AlCl3 | FeCl3 | MgCl2 |
�е�/�� | 58 | 136 | 181�������� | 316 | 1412 |
�۵�/�� | 69 | 25 | 193 | 304 | 714 |
��TiCl4�е��ܽ��� | ���� | ���� | �� | ���� | |
��1���Ȼ����̣�TiO2��Cl2����ֱ�ӷ�Ӧ����̼����CO��CO2��ʹ��Ӧ���Խ��С�
��֪��TiO2(s)+2 Cl2(g)= TiCl4(g)+ O2(g) ��H1=+175.4 kJ��mol-1
2C(s)+O2(g)=2CO(g) ��H2=-220.9 kJ��mol-1
�� ����¯�м�̼�Ȼ�����TiCl4(g)��CO(g)���Ȼ�ѧ����ʽ��____________________��
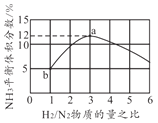
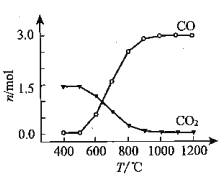
�� �Ȼ�������CO��CO2�����ת����������ͼ�жϣ�CO2����CO��Ӧ�Ħ�H_____0���������������=�������ж����ݣ�_______________��
�� �Ȼ���Ӧ��β���봦�����ŷţ�β���е�HCl��Cl2�����տɵô����ᡢFeCl3��Һ����β��������Һ������__________________________��
�� �Ȼ�������ȴ�����£������˵õ���TiCl4���Һ���������к���_____________��
��2�����ƹ��̣���TiCl4����������ô�TiCl4��ʾ��ͼ���£�
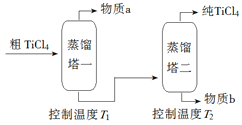
����a��______________��T2Ӧ������_________��