��Ŀ����
(18��)�ס������ص缫���϶���������̼������ش��������⣺

(1)�������о�ΪCuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е�________�����ҳ��е�________����
���ҳ��������ĵ缫��Ӧʽ��_______________________________________________ _
(2)�������о�Ϊ����NaCl��Һ��
���ҳ���̼���ϵ缫��Ӧ����____________(�������Ӧ����ԭ��Ӧ��)��д���ҳ��иõ缫��Ӧ�����ӷ���ʽ_______________________________________��
�ڼ׳���̼���ϵ缫��Ӧʽ��_____________������ø�����Ӧ����ķ�����_____________________________�����ӷ���ʽ��ʾ_______________________________
�����ҳ�ת��0.02 mol e����ֹͣʵ�飬������Һ�����200 mL������Һ���Ⱥ��pH��________��

(1)�������о�ΪCuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е�________�����ҳ��е�________����
���ҳ��������ĵ缫��Ӧʽ��_______________________________________________ _
(2)�������о�Ϊ����NaCl��Һ��
���ҳ���̼���ϵ缫��Ӧ����____________(�������Ӧ����ԭ��Ӧ��)��д���ҳ��иõ缫��Ӧ�����ӷ���ʽ_______________________________________��
�ڼ׳���̼���ϵ缫��Ӧʽ��_____________������ø�����Ӧ����ķ�����_____________________________�����ӷ���ʽ��ʾ_______________________________
�����ҳ�ת��0.02 mol e����ֹͣʵ�飬������Һ�����200 mL������Һ���Ⱥ��pH��________��
(1)��̼����
��4OH����4e��===2H2O��O2��
(2)�� ������Ӧ 2Cl����2e��=Cl2����
��2H2O��O2��4e��
 =4OH�����μ�2�����軯����Һ����������ɫ�������֣�˵��������Fe2�� 3Fe2�� +2[Fe(CN)6]3-
=4OH�����μ�2�����軯����Һ����������ɫ�������֣�˵��������Fe2�� 3Fe2�� +2[Fe(CN)6]3-  = Fe3[Fe(CN)6]2����ɫ�������������³ʿ��
= Fe3[Fe(CN)6]2����ɫ�������������³ʿ����13
(1)��̼��������������������
������Ϊ̼����ʧ���ӣ�4OH����4e��===2H2O��O2��
(2)�� ������Ӧ 2Cl����2e��=Cl2����
��2H2O��O2��4e��
 =4OH�����μ�2�����軯����Һ����������ɫ�������֣�˵��������Fe2�� 3Fe2�� +2[Fe(CN)6]3-
=4OH�����μ�2�����軯����Һ����������ɫ�������֣�˵��������Fe2�� 3Fe2�� +2[Fe(CN)6]3-  = Fe3[Fe(CN)6]2����ɫ�������������³ʿ��
= Fe3[Fe(CN)6]2����ɫ�������������³ʿ����H�� Ũ��Ϊ��0.02mol/0.200L=0.1mol/L,pH= 13

��ϰ��ϵ�д�
�����Ŀ
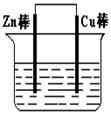
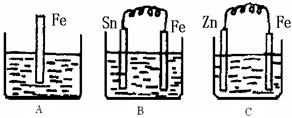
 2Fe2����I2��Ƴ�����ͼ��ʾ��ԭ��ء������жϲ���ȷ����
2Fe2����I2��Ƴ�����ͼ��ʾ��ԭ��ء������жϲ���ȷ����

