��Ŀ����
���û�ѧԭ�����ԶԹ����ŷŵķ�ˮ�������Ƚ�����Ч��������������ij�������Ƹ﹤ҵ������Cr(III)�Ĵ����������£�
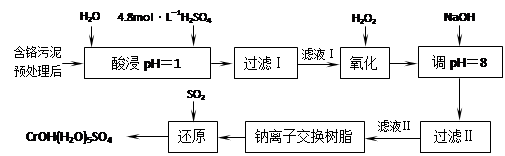
���������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+��
��1��ʵ������18.4 mol��L�D1��Ũ��������250 mL 4.8 mol��L�D1��������Һ�����õIJ����������ձ����������ͽ�ͷ�ι��⣬���� ��
��2�����ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ�� ��
��(д��������ʩ)
��3��H2O2�������ǽ���Һ���е�Cr3+ת��ΪCr2O72�D��д���˷�Ӧ�����ӷ���ʽ��
��
��4�������£�����������������������ʽ����ʱ��Һ��pH���£�
����NaOH��Һʹ��Һ�ʼ��ԣ�Cr2O72�Dת��ΪCrO42�D����Һ������������Ҫ�� ������Һ��pH���ܳ���8���������� ��
��5�������ӽ�����֬�ķ�Ӧԭ��Ϊ��Mn+��nNaR��MRn��nNa+�����������ӽ�����֬��ȥ��Һ���еĽ����������� ��
��6��д��������������SO2���л�ԭ�Ļ�ѧ����ʽ ��
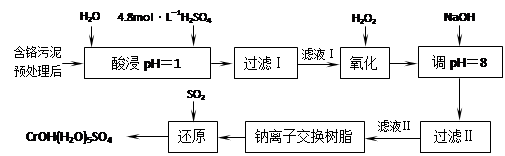
���������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+��
��1��ʵ������18.4 mol��L�D1��Ũ��������250 mL 4.8 mol��L�D1��������Һ�����õIJ����������ձ����������ͽ�ͷ�ι��⣬���� ��
��2�����ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ�� ��
��(д��������ʩ)
��3��H2O2�������ǽ���Һ���е�Cr3+ת��ΪCr2O72�D��д���˷�Ӧ�����ӷ���ʽ��
��
��4�������£�����������������������ʽ����ʱ��Һ��pH���£�
| ������ | Fe3+ | Mg2+ | Al3+ | Cr3+ |
| ��ʼ����ʱ��pH | 2.7 | ���� | ���� | ���� |
| ������ȫʱ��pH | 3.7 | 11.1 | 8 | 9����9�ܽ⣩ |
����NaOH��Һʹ��Һ�ʼ��ԣ�Cr2O72�Dת��ΪCrO42�D����Һ������������Ҫ�� ������Һ��pH���ܳ���8���������� ��
��5�������ӽ�����֬�ķ�Ӧԭ��Ϊ��Mn+��nNaR��MRn��nNa+�����������ӽ�����֬��ȥ��Һ���еĽ����������� ��
��6��д��������������SO2���л�ԭ�Ļ�ѧ����ʽ ��
��1��250mL����ƿ��1�֣�����Ͳ��1�֣�
��2�����߷�Ӧ�¶ȡ������������ı�������ӿ�����ٶȵȣ�3���д�2�㼴��2�֣�
��3��2Cr3+ + 3H2O2 + H2O �� Cr2O72��+ 8H+��2�֣�
��4��Na+��Mg2+��Ca2+��2�֣�
pH����8��ʹ����Al(OH)3�ܽ�����AlO2��������Ӱ��Cr(III)�����������ã�2�֣�
��5��Ca2+��Mg2+��2�֣�
��6��3SO2 + 2Na2CrO4 + 12H2O �� 2CrOH(H2O)5SO4��+ Na2SO4 + 2NaOH ��2�֣�
��3SO2 + 2CrO42�� + 12H2O �� 2CrOH(H2O)5SO4��+ SO42�� + 2OH����
��2�����߷�Ӧ�¶ȡ������������ı�������ӿ�����ٶȵȣ�3���д�2�㼴��2�֣�
��3��2Cr3+ + 3H2O2 + H2O �� Cr2O72��+ 8H+��2�֣�
��4��Na+��Mg2+��Ca2+��2�֣�
pH����8��ʹ����Al(OH)3�ܽ�����AlO2��������Ӱ��Cr(III)�����������ã�2�֣�
��5��Ca2+��Mg2+��2�֣�
��6��3SO2 + 2Na2CrO4 + 12H2O �� 2CrOH(H2O)5SO4��+ Na2SO4 + 2NaOH ��2�֣�
��3SO2 + 2CrO42�� + 12H2O �� 2CrOH(H2O)5SO4��+ SO42�� + 2OH����
��1���������ʵ���Ũ�ȵ����ơ�����������������֪����ȱ��250ml����ƿ����Ͳ��
��2��������������Է�Ӧ���ʵ�Ӱ�졣�ɴ��¶ȣ�Ũ�ȺͽӴ�����ȽǶȽ��п��ǡ�
��3������������ԭ��Ӧ����ʽ����ƽ�����ݵ�ʧ�����غ������ƽ��
��4�����ݳ���ʱ��pHֵ�����жϣ���pH��8ʱ����Һ�е�Fe3+��Al3+�Ѿ�����������������γ���������Һ�е���������Ҫ����Na+��Mg2+��Ca2+����ΪpH̫�ߣ����ܽ���������������Ӱ�������ʵ��ķ�����ᴿ��
��5�����������ӽ�����֬�ķ�Ӧԭ����֪����Һ�е�Ca2+��Mg2+������������ȥ��
��6���������ʷ�Ӧǰ��ı仯��֪����������������ԭ��Һ�е�CrO42�D���仹ԭ������CrOH(H2O)5SO4��Ȼ����ݵ�ʧ�����غ���ƽ���ɡ�
��2��������������Է�Ӧ���ʵ�Ӱ�졣�ɴ��¶ȣ�Ũ�ȺͽӴ�����ȽǶȽ��п��ǡ�
��3������������ԭ��Ӧ����ʽ����ƽ�����ݵ�ʧ�����غ������ƽ��
��4�����ݳ���ʱ��pHֵ�����жϣ���pH��8ʱ����Һ�е�Fe3+��Al3+�Ѿ�����������������γ���������Һ�е���������Ҫ����Na+��Mg2+��Ca2+����ΪpH̫�ߣ����ܽ���������������Ӱ�������ʵ��ķ�����ᴿ��
��5�����������ӽ�����֬�ķ�Ӧԭ����֪����Һ�е�Ca2+��Mg2+������������ȥ��
��6���������ʷ�Ӧǰ��ı仯��֪����������������ԭ��Һ�е�CrO42�D���仹ԭ������CrOH(H2O)5SO4��Ȼ����ݵ�ʧ�����غ���ƽ���ɡ�

��ϰ��ϵ�д�
�����Ŀ
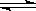 H3O++OH-
H3O++OH-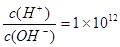 ���Һ��д���Al3+��NO3�����������Һ���Ƿ������������6 �����ӣ���SiO32����NH4����Fe2+ ��Na+ ��HCO3����Cl�������в��ؼ�����ܼ��Է������ǣ� ��
���Һ��д���Al3+��NO3�����������Һ���Ƿ������������6 �����ӣ���SiO32����NH4����Fe2+ ��Na+ ��HCO3����Cl�������в��ؼ�����ܼ��Է������ǣ� �� ����Һ��
����Һ��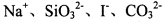
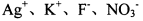
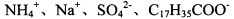
 ��NaHSO4��Һ��
��NaHSO4��Һ��