��Ŀ����
����Ŀ������˵����ȷ����( )
A. ��ϵͳ�����������л���![]() ��
�� ������̼ԭ������Ϊ7��
������̼ԭ������Ϊ7��
B. ij���ķ���ʽΪC10H14��������ʹ��ˮ��ɫ������ʹ����KMnO4��Һ��ɫ���ҷ��ӽṹ��ֻ��һ���������������������4��
C. ̼ԭ����С�ڻ����8�ĵ�ϩ���У���HBr�ӳɲ���ֻ��һ�ֽṹ�����������ĵ�ϩ����6��
D. ���ⶨC3H7OH��C6H12��ɵĻ������������������Ϊ8%����˻������̼������������78%
���𰸡�D
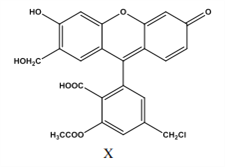
�����������������A��![]() ��������8��̼ԭ�ӣ���A���� B�������ķ���ʽ����CnH2n-6��ͨʽ��������ʹ��ˮ��ɫ������ʹKMnO4������Һ��ɫ�����Ժ��б�������������֪���÷��Ӻ��ж������������ֻ�ж϶��������ͬ���칹�弴�ɣ����������̼��ͬ���칹����-��CH2��2CH3��-CH2CH��CH3��2��-CH��CH3��CH2CH3��-C��CH3��3������ͬϵ�����뱽��������Cԭ���ϱ��뺬��Hԭ�ӣ��ſɱ����Ը������������ʹ���Ը��������Һ��ɫ�����-C��CH3��3�뱽��������Cԭ���ϲ���Hԭ�ӣ�����ʹ���Ը��������Һ��ɫ����������������3�֣���B����C�����������ĵ�ϩ����4�֣�̼ԭ����С��8�ĵ�ϩ����HBr�����ӳɷ�Ӧֻ��һ�ֲ��˵���õ�ϩ������̼̼˫��Ϊ���ĵĶԳƽṹ��������һ�������У�CH2�TCH2��CH3-CH�TCH-CH3��CH3-CH2-CH�TCH-CH2-CH3����CH3��2C=C��CH3��2����C����D��C3H7OH��O����������Ϊ
��������8��̼ԭ�ӣ���A���� B�������ķ���ʽ����CnH2n-6��ͨʽ��������ʹ��ˮ��ɫ������ʹKMnO4������Һ��ɫ�����Ժ��б�������������֪���÷��Ӻ��ж������������ֻ�ж϶��������ͬ���칹�弴�ɣ����������̼��ͬ���칹����-��CH2��2CH3��-CH2CH��CH3��2��-CH��CH3��CH2CH3��-C��CH3��3������ͬϵ�����뱽��������Cԭ���ϱ��뺬��Hԭ�ӣ��ſɱ����Ը������������ʹ���Ը��������Һ��ɫ�����-C��CH3��3�뱽��������Cԭ���ϲ���Hԭ�ӣ�����ʹ���Ը��������Һ��ɫ����������������3�֣���B����C�����������ĵ�ϩ����4�֣�̼ԭ����С��8�ĵ�ϩ����HBr�����ӳɷ�Ӧֻ��һ�ֲ��˵���õ�ϩ������̼̼˫��Ϊ���ĵĶԳƽṹ��������һ�������У�CH2�TCH2��CH3-CH�TCH-CH3��CH3-CH2-CH�TCH-CH2-CH3����CH3��2C=C��CH3��2����C����D��C3H7OH��O����������Ϊ![]() �����Ի������C3H7OH��������Ϊ
�����Ի������C3H7OH��������Ϊ![]() =30%�����Ի������C6H12����������Ϊ1-30%=70%���������C3H7OH��HԪ���ڻ�����е���������Ϊ
=30%�����Ի������C6H12����������Ϊ1-30%=70%���������C3H7OH��HԪ���ڻ�����е���������Ϊ![]() ��30%=4%���������C6H12��HԪ���ڻ�����е���������Ϊ
��30%=4%���������C6H12��HԪ���ڻ�����е���������Ϊ![]() ��70%=10%�����Ի�����������������ΪH%=4%+10%=14%���������C����������Ϊ1-14%-8%=78%����D��ȷ����ѡD��
��70%=10%�����Ի�����������������ΪH%=4%+10%=14%���������C����������Ϊ1-14%-8%=78%����D��ȷ����ѡD��

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�����Ŀ�����������й�����������Һ��������ȷ����(������Һ���ʱ����仯)
�� | �� | �� | �� | |
pH | 12 | 12 | 2 | 2 |
��Һ | ��ˮ | ����������Һ | ���� | ���� |
A. ���٢��зֱ�����Ȼ�茶���������Һ��pHֵ������
B. �ֱ��������������ˮϡ��100����������Һ��pH����>��
C. ���٢�����Һ�������Ϻ�������Һ������
D. ����Һ������Һ��������������Ϻ�������ҺpH��7