��Ŀ����
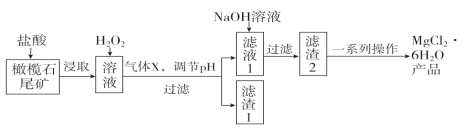
����Ŀ��ʵ�����������ʯβ��(��Ҫ�ɷ�ΪMgO������FeO��Fe2O3��Al2O3��)�Ʊ������Ȼ�þ����(MgCl2��6H2O)��ʵ���������£�
��֪���ֽ����������γ������������ʱ��pH���±���
Fe2+ | Fe3+ | Al3+ | Mg2+ | |
��ʼ����ʱ | 7.6 | 2.7 | 4.2 | 9.6 |
������ȫʱ | 9.6 | 3.7 | 5.4 | 11.1 |
�ش��������⣺
(1)����ȡ�������У��ܼӿ��ȡ���ʵķ�����____________��__________(��д����)��
(2)����X�ĵ���ʽΪ________������1�������������Ƶ�һ�ָ�Ч�����߷��ӻ���������ˮ�����仯ѧʽΪ[Fe2(OH)n(SO4)(3��0.5n)]m�������������Ԫ�صĻ��ϼ�Ϊ________��
(3)����H2O2��Ŀ����______________________���������������е���H2O2������NaClO������Ҳ�ܴﵽͬ��Ŀ�ģ�������Ӧ�����ӷ���ʽΪ��________________________________��
(4)��һϵ�в�������Ҫ���������������ᣬȻ��____________________�����ˡ�ϴ�ӣ����õ��Ȼ�þ���塣
(5)ȷ��ȡ2.000 g�Ȼ�þ�����Ʒ��250 mL��ƿ�У���ˮ50 mLʹ����ȫ�ܽ⣬����100 mL���Ի���Һ����������Tָʾ������Һ�Ծƺ�ɫ���ڲ������£���0.5000 mol/L��EDTA����Һ���еζ����䷴Ӧԭ��ΪMg2+��Y4- ==MgY2-���ζ��յ�ʱ����EDTA����Һ�����19.00 mL��
�����Ʒ��MgCl2��6H2O����������Ϊ________(���������λ��Ч����)��
�����еζ������ᵼ�²������ƫ�ߵ���________(����ĸ)��
a���ζ��յ�ʱ���Ӷ��� b����ƿϴ�Ӻ�û�и���
c���ζ�ʱ��ƿ����Һ�彦�� d���ζ��ܵζ�ǰ�����ݣ��ζ���������ʧ
���𰸡������ʯβ����顢��������Ũ�ȡ��ʵ���߷�Ӧ�¶ȵ� ![]() +3 ��Fe2+����ΪFe3+ ClO-��2Fe2+��2H+=2Fe3+��Cl-��H2O ����Ũ�� ��ȴ�ᾧ 96.4% ad
+3 ��Fe2+����ΪFe3+ ClO-��2Fe2+��2H+=2Fe3+��Cl-��H2O ����Ũ�� ��ȴ�ᾧ 96.4% ad
��������
������ȡ�������У��ܼӿ��ȡ���ʵķ����н����ʯβ����顢��������Ũ�ȡ��ʵ���߷�Ӧ�¶ȵȡ�
��X����ͨ�������Һ��pHֵ����Ҫ�ǰ��������ݻ��ϼ۷����ó�����Ԫ�صĻ��ϼۡ�
�Ǽ���H2O2��Ŀ���ǽ�Fe2+����ΪFe3+���Ա������Ԫ�أ�����NaClO��������H2O2��Ҳ�ܴﵽͬ��Ŀ�Ľ�����д���ӷ���ʽ��
����һϵ�в�������Ҫ���������������ᣬȻ������Ũ�������ˡ�ϴ�ӡ�
���ȼ���MgCl2��6H2O���ʵ���Ϊ0.5000mol/L��0.019L =0.0095mol���ټ������������������к͵ζ�ԭ�����з�����
������ȡ�������У��ܼӿ��ȡ���ʵķ����н����ʯβ����顢��������Ũ�ȡ��ʵ���߷�Ӧ�¶ȵȣ��ʴ�Ϊ�������ʯβ����顢��������Ũ�ȡ��ʵ���߷�Ӧ�¶ȵȡ�
��X����ͨ�������Һ��pHֵ�����Ϊ�����������ĵ���ʽΪ![]() ������1��ѧʽΪ[Fe2(OH)n(SO4)(3��0.5n)]m�����ݻ��ϼ۷����ó�����Ԫ�صĻ��ϼ�Ϊ2x + (-1)��n + (-2)��(3-0.5n) = 0��x = +3���ʴ�Ϊ��+3��
������1��ѧʽΪ[Fe2(OH)n(SO4)(3��0.5n)]m�����ݻ��ϼ۷����ó�����Ԫ�صĻ��ϼ�Ϊ2x + (-1)��n + (-2)��(3-0.5n) = 0��x = +3���ʴ�Ϊ��+3��
�Ǽ���H2O2��Ŀ���ǽ�Fe2+����ΪFe3+���Ա������Ԫ�أ��������������е���H2O2������NaClO������Ҳ�ܴﵽͬ��Ŀ�ģ�������Ӧ�����ӷ���ʽΪ��ClO����2Fe2+��2H+=2Fe3+��Cl����H2O���ʴ�Ϊ����Fe2+����ΪFe3+��ClO����2Fe2+��2H+=2Fe3+��Cl����H2O��
����һϵ�в�������Ҫ���������������ᣬȻ������Ũ�������ˡ�ϴ�ӣ����õ��Ȼ�þ���壬�ʴ�Ϊ������Ũ����
�������ݷ�Ӧԭ���õ���Ʒ��MgCl2��6H2O���ʵ���Ϊ0.5000mol/L��0.019L =0.0095mol������������Ϊ![]() ���ʴ�Ϊ��96.4%��
���ʴ�Ϊ��96.4%��
��aѡ��ζ��յ�ʱ���Ӷ���������ƫ�ⶨ���ƫ�ߣ���a�������⣻
bѡ���ƿϴ�Ӻ�û�и��û��Ӱ�죬��b���������⣻
cѡ��ζ�ʱ��ƿ����Һ�彦��������Һ���ʼ��٣����ı�Һ���٣�������٣����ƫ�ͣ���c���������⣻
dѡ��ζ��ܵζ�ǰ�����ݣ��ζ���������ʧ���������ƫ�ⶨ���ƫ�ߣ���d�������⡣
������������Ϊad��

����Ŀ����1����д���пո�
���� | ������ | ������ |
___ | 1 | 0 |
| ___ | ___ |
| ___ | b |
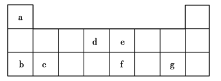
��2��Ԫ�����ڱ��е������ڹ���___��Ԫ�أ����������ڢ�A��Ԫ�ص�ԭ������Ϊ![]() ����������ڢ�A��Ԫ�ص�ԭ������Ϊ___���ú�
����������ڢ�A��Ԫ�ص�ԭ������Ϊ___���ú�![]() �Ĵ���ʽ��ʾ����ͬ�����������ڢ�A��Ԫ�ص�ԭ������Ϊ____��
�Ĵ���ʽ��ʾ����ͬ�����������ڢ�A��Ԫ�ص�ԭ������Ϊ____��
��3�����ڵڢ���֮���һ������Ϊ��____�壬Ԫ�����ڱ�������Ԫ�������������ǵ�___�塣
��4��ijԪ�ص�һ��ͬλ��![]() ��ԭ�ӵ�������Ϊ
��ԭ�ӵ�������Ϊ![]() ����
����![]() �����ӣ�����
�����ӣ�����![]() ԭ�����
ԭ�����![]() ���ӣ���
���ӣ���![]()
![]()
![]() ���������ӵ����ʵ�����___
���������ӵ����ʵ�����___![]() ��
��
����Ŀ����ǹ�ҵ���Ʊ�Na2S2O3�ķ���֮һ����Ӧԭ��Ϊ��2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2���÷�Ӧ��>0��ij�о�С����ʵ��������Ʊ�Na2S2O3��5H2O�������¡�

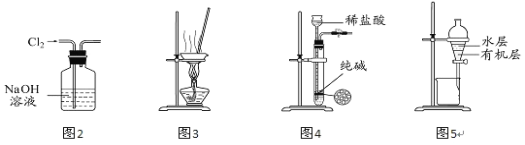
��1������װ����ͼ��ʾ��

��װ��B�������Ǽ���װ����SO2������Ч�ʣ�B���Լ���________������SO2����Ч�ʵ͵�ʵ��������B����Һ________________��
��Ϊ��ʹSO2������������ȫ���ڲ��ı�A����ҺŨ�ȡ�����������£����˼�ʱ���跴Ӧ���⣬���ɲ�ȡ�ĺ�����ʩ��_______��_______����д��������
��2�����豾ʵ�����õ�Na2CO3������NaCl��NaOH�����ʵ�鷽�����м��顣������ʱCaCO3������Һ��pH=12��, �����Լ���������ϡ���ᡢAgNO3��Һ��CaCl2��Һ��Ca(NO3)2��Һ����̪��Һ������ˮ��pH�ơ��ձ����Թܡ��ιܡ�
��� | ʵ����� | Ԥ������ | ���� |
�� | ȡ������Ʒ���Թ��У�������������ˮ��������ܽ⣬_________�� | �а�ɫ�������� | ��Ʒ��NaCl |
�� | ��ȡ������Ʒ���ձ��У�������������ˮ��������ܽ⣬_________�� | �а�ɫ�������ɣ��ϲ���ҺpH>10.2 | ��Ʒ��NaOH |
��3��Na2S2O3��Һ�Ƕ���ʵ���еij����Լ����ⶨ��Ũ�ȵĹ������£�
��һ����ȷ��ȡag KIO3����Է���������214�����������Һ��
�ڶ������������KI�����H2SO4��Һ���μ�ָʾ����
����������Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��Һ��Һ�����ΪvmL ��c(Na2S2O3��Һ)��_______mol��L-1����ֻ�г���ʽ���������㣩
��֪��IO3-��I-��6H+=3I2+3H2O ��2S2O32-��I2=S4O62-��2I- ijͬѧ��һ���͵ڶ����IJ������ܹ淶������������̫����������õ�Na2S2O3��Ũ�ȿ���_____�������Ӱ�족����ƫ�͡���ƫ�ߡ�) ��ԭ����________�������ӷ���ʽ��ʾ����