��Ŀ����
����Ŀ��������Ҫ�Ľ������ϣ���������Ҫ�ɷ�A12O3����������SiO2��Fe2O3�ȣ��ǹ�ҵ��������ԭ�ϡ�ʵ����ģ�ҵ����������Ϊԭ����ȡ���������[NH4Al��SO4��2��12H2O]�Ĺ�����������ͼ��ʾ��

��ش��������⣺
��1��Ϊ���������������ķ�Ӧ���ʣ��ɲ�ȡ�Ĵ�ʩ��______________________________��д��������
��2������a����Ҫ�ɷ�Ϊ__________________��д��ѧʽ����
��3��II���ռ������ԭ����________________________________________��
��4��д��III����Ҫ��Ӧ�����ӷ���ʽ__________________________________________��
��5����ȡ�������Һ�Ļ�ѧ����ʽΪ_________________________________________�����������Һ��ȡ����������ʵ���������Ϊ�������������______________________��____________�����ˡ�ϴ�ӡ�
��6�����ʵ��֤���������Һ�к���NH4+_______________________________________��
���𰸡� ���ȡ���������Ũ�ȡ������������� SiO2 ʹAl3+ȫ��ת��ΪAlO2�� AlO2����CO2��2H2O��Al��OH�� 3����HCO3�� Al2O3��4H2SO4��2NH3��2NH4Al��SO4��2��3H2O��Al2��SO4��3��H2SO4��2NH3��2NH4Al��SO4��2 ����Ũ�� ��ȴ�ᾧ ȡ�����������Һ��һ֧�ྻ���Թ��У��ټ���Ũ����������Һ�����ȣ����������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤���������Һ�к���NH4+
����������������Al2O3��Fe2O3���������ᣬSiO2���������ᣬ���Թ���aΪSiO2���������������������Һ����ƫ�����ƺ�����������������b������������ͨ������CO2����Al��OH��3��������Һc��̼�����������������ֽ���������������V������������ϡ���ᷴӦ������������ͨ�백�������������Һ�����������Һ�л������������ʵ���������Ϊ����Ũ������ȴ�ᾧ��������ϴ�ӵ�����
��1��������������Է�Ӧ���ʵ�Ӱ���֪Ϊ���������������ķ�Ӧ���ʣ��ɲ�ȡ�Ĵ�ʩ�м��ȡ���������Ũ�ȡ������������ȡ���2���������Ϸ�����֪����a����Ҫ�ɷ�ΪSiO2����3���������Ϸ�����֪II���ռ������ԭ����ʹAl3+ȫ��ת��ΪAlO2������4���������Ϸ�����֪III����Ҫ��Ӧ�����ӷ���ʽΪAlO2����CO2��2H2O��Al��OH��3����HCO3������5������ԭ���غ��֪��ȡ�������Һ�Ļ�ѧ����ʽΪAl2��SO4��3��H2SO4��2NH3��2NH4Al��SO4��2���������Ϸ�����֪���������Һ��ȡ����������ʵ���������Ϊ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ���6��笠�����ǿ�Ӧ�����������就������˼����������Һ�к���NH4+��ʵ�����Ϊȡ�����������Һ��һ֧�ྻ���Թ��У��ټ���Ũ����������Һ�����ȣ����������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤���������Һ�к���NH4+��

 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д�����Ŀ����10 L�����ܱ������г���X(g)��Y(g)��������ӦX(g)��Y(g) ![]() M(g)��N(g)������ʵ���������±���
M(g)��N(g)������ʵ���������±���
ʵ�� ��� | �¶�/�� | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | ||
n(X) | n(Y) | n(M) | |||
�� | 700 | 0.40 | 0.10 | 0.090 | |
�� | 800 | 0.10 | 0.40 | 0.080 | |
�� | 800 | 0.20 | 0.30 | a | |
�� | 900 | 0.10 | 0.15 | b | |
����˵����ȷ����(����)
A. ʵ�����У���5 minʱ���n(M)��0.050 mol����0��5 minʱ���ڣ���N��ʾ��ƽ����Ӧ����v(N)��1.0��10��2 mol��L��1��min��1
B. ʵ�����У��÷�Ӧ��ƽ�ⳣ��K��2.0
C. ʵ�����У��ﵽƽ��ʱ��X��ת����Ϊ60%
D. ʵ�����У��ﵽƽ��ʱ��b>0.060
����Ŀ��һ����Һ�п��ܴ�������K+��A13+��H+��NH4+��Cl-��Br-��I-��ClO-��A1O2-�������е������֡�Ϊ��ȷ����Һ����ɣ����������²�����
��1��������Һ����μ���NaOH��Һ���ʵ����ȣ�������������������ʵ���(n)�����NaOH��Һ�������ϵ��ͼ��ʾ��
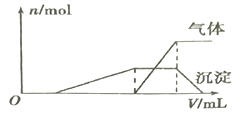
�����Һ��һ�����ڵ�������________��һ�����������ڵ�������__________��
��2����������Һ�к��д�����Cl-��Br-��I-������1L�û����Һ��ͨ��һ����������������Һ��Cl-��Br-��I-����ͨ�����������(��״����)��ϵ���±���ʾ���ش��������⣺
Cl2�����(��״����) | 2.8 L | 5.6 L | 11.2 L |
n(Cl-) | 1.25 mol | 1.5 mol | 2 mol |
n(Br-) | 1.5 mol | 1.4 mol | 0.9 mol |
n(I-) | a mol | 0 | 0 |
��ͨ�������Ϊ2.8L(��״����)ʱ����Һ�з�����Ӧ�����ӷ���ʽΪ___________��ԭ��Һ��n(Cl-)Ϊ____mol��ͨ�������������2.8L��5.6L(��״����)֮��ʱ���й����ӷ���ʽΪ(���ж����Ӧ����ֿ���д)_________________��ԭ��Һ��Cl-��Br-��I-�����ʵ���Ũ��֮��Ϊ_______________��
