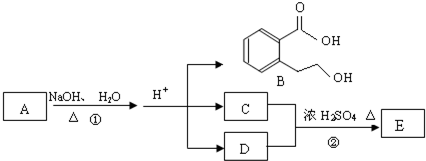
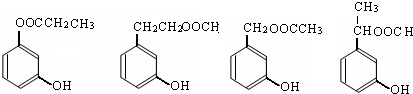
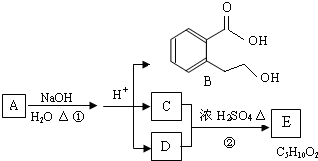
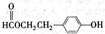��Ŀ����
A��B��C��D��E��Ϊ���Σ�D��E������ͬ��Ԫ����ɣ�����������Ԫ����ɵĻ������B��Һ�еμ������ữ����������Һ������������ɫ�������ó�������䰵�������ڡ�A��B��B��C��Һ��Ͼ���������������A��B��E��B��C��E�ֱ�1:5:6��1:1:2�����ʵ���֮�Ȼ�ϣ���Һ���ܲ����д̼�����ζ����ɫ����ס�D��E��Һ����ܲ�������Ư���ԵĴ̼��������ҡ������һ������ͨ��ˮ�л���������ᡣB��E�����ϲ���Ӧ���������ܲ����д̼�����ζ������������������ͨ��A��C��Һ�о��ܲ�������ס�������ͨ��D��Һ����ɫ����ʧ������A��D��E��2:1:1���ʵ���֮�Ȼ�ϣ��ܲ�����ɫ���嶡��
��ش������й����⣺
�ţ�������з�Ӧ�����ӷ���ʽ����ƽ֮��
�٣�A + B + E �� ���� ��
�ڣ�D + E �� ���� ��
�ۣ���+��+ H2O �� �� ��
�ܣ�A + D + E �� ���� ��
�ƣ�����������Ư���Աȼ�ǿ���Ǹ������Ư���Լ�����ͬ�����£���ͬ����Ķ����崦��ˮ�������Ǽ� �� ������ͬ�����Ķ����崦��ˮ�������Ǽ� �� ����
�ǣ���ʷ������ijԪ�ص���۵ĺ������Ϊij�ᣬ��N��P��S�ȡ�A���ж�Ӧ���Ტ���Ǹ�Ԫ�ص���۵ĺ����ᣨ��ʱ����Ϊ����ۣ�����⼼�����뻯ѧ�о������ͨ�����Բ��ϵ��A��ˮ��Һ�������µĺ�������(M=122.5 g��mol��1)�����ǿ��������Ӧ�����Ϊ��ij�ᡣȡ��100 mL A����Һ���е�⣨���Ե缫��ֱͨ���磩����Сʱ���ռ�������������������ֱ�Ϊ6.72L��2.24L(�Ѿ�����ɱ�״���µ��������) ��
�٣������������������ĵ缫��Ӧ���ӷ���ʽΪ �� �� �� ��
�ڣ�ԭA����Һ�����ʵ���Ũ��Ϊ �� ����������Һ����仯���Բ��ƣ�
�ţ� ��ClO![]() + 5Cl��+ 6H+ = 3Cl2 + 3H2O ��HSO
+ 5Cl��+ 6H+ = 3Cl2 + 3H2O ��HSO![]() + H+ = SO2 �� + H2O
+ H+ = SO2 �� + H2O
��Cl2��SO2 + 2H2O = 4H+ + 2Cl�� + SO![]() ��2ClO
��2ClO![]() + HSO
+ HSO![]() + H+=2ClO2 ��+ SO
+ H+=2ClO2 ��+ SO![]() + H2O
+ H2O
�ƣ� 2.5 ���� 2.63��
�ǣ� ��������2H2O + 2e�� = 2OH�� + H2 ����2H++2e�� = H2������
��������ClO![]() -2e�� + H2O = ClO
-2e�� + H2O = ClO![]() + 2H+����2H2O-4e��=O2��+4H+��4OH��-4e��= O2��+2H2O
+ 2H+����2H2O-4e��=O2��+4H+��4OH��-4e��= O2��+2H2O
�� 1 mol��L��1��