��Ŀ����
����Ŀ����CH3CH=CH2��NH3��O2Ϊԭ�ϣ��ڴ������������ɱ�ϩ��(C3H3N)�������ϩȩ(C3H4O)�Ļ�ѧ����ʽ�ֱ�Ϊ����Ӧ��.2C3H6+2NH3+3O2![]() 2C3H3N(g)+6H2O(g)����Ӧ��.C3H6+O2
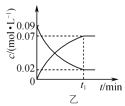
2C3H3N(g)+6H2O(g)����Ӧ��.C3H6+O2![]() C3H4O(g)+H2O(g)����Ӧ��ͬʱ�䣬��ϩ������뷴Ӧ�¶ȵĹ�ϵ��ͼa��ʾ����ϩ��ͱ�ϩȩ��ƽ�������
C3H4O(g)+H2O(g)����Ӧ��ͬʱ�䣬��ϩ������뷴Ӧ�¶ȵĹ�ϵ��ͼa��ʾ����ϩ��ͱ�ϩȩ��ƽ�������![]() �Ĺ�ϵ��ͼb��ʾ������˵����ȷ����(��ϩ���ѡ����=
�Ĺ�ϵ��ͼb��ʾ������˵����ȷ����(��ϩ���ѡ����=![]() ��100%)�� ��
��100%)�� ��
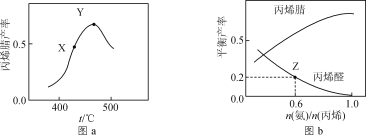
A.�����������䣬����ѹǿ��������߱�ϩ��ƽ�����
B.ͼa��X����ʾ�����£��ӳ���Ӧʱ������߱�ϩ�����
C.ͼa��Y����ʾ�����£����öԱ�ϩ��ѡ���Ը��õĴ�������߱�ϩ�����
D.��ͼb��Z���֪�����¶��·�Ӧ���ƽ�ⳣ��ΪK=![]()
���𰸡�BC
��������
A��2C3H6+2NH3+3O2![]() 2C3H3N(g)+6H2O(g)�������������ķ�Ӧ��������ѹǿƽ���� ���ƶ�����ϩ��ƽ����ʽ��ͣ�A����
2C3H3N(g)+6H2O(g)�������������ķ�Ӧ��������ѹǿƽ���� ���ƶ�����ϩ��ƽ����ʽ��ͣ�A����
B����ͼ��֪��X���ϩ��IJ���δ�ﵽ���ֵ��������X��������£��ӳ���Ӧʱ������߱�ϩ��IJ��ʣ�B��ȷ��
C��Y���ϩ��IJ��ʴﵽ���ֵ�����öԱ�ϩ��ѡ���Ը��õĴ������������ӱ�ϩ��IJ��ʣ����ٸ����������C��ȷ��
D��![]() ��100%����ͼb�п��Կ�����ϩȩ��ƽ�������0.2������Ŀû�и��߱�ϩ����ʼ���Լ�ת�����Ƕ��٣������㷴Ӧ���ϩ�Լ���ϩȩ��Ũ�ȣ�����ƽ�����ʽ
��100%����ͼb�п��Կ�����ϩȩ��ƽ�������0.2������Ŀû�и��߱�ϩ����ʼ���Լ�ת�����Ƕ��٣������㷴Ӧ���ϩ�Լ���ϩȩ��Ũ�ȣ�����ƽ�����ʽ![]() ���ĸ����ʵ�Ũ�Ⱦ�Ϊδ֪����D����
���ĸ����ʵ�Ũ�Ⱦ�Ϊδ֪����D����
��ѡBC��

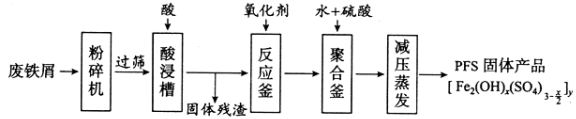
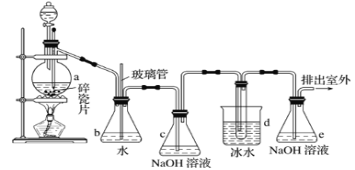
����Ŀ�������������Ӽ�����1��2-�������顣��ͼΪʵ�����Ʊ�1��2-���������װ�Dͼ�� ͼ�з�Һ©������ƿa�зֱ�װ��ŨH2SO4����ˮ�Ҵ���dװ�D�Թ���װ��Һ�塣
��֪��CH3CH2OH![]() CH2=CH2��+H2O��
CH2=CH2��+H2O��
2CH3CH2OH![]() CH3CH2OCH2CH3+H2O
CH3CH2OCH2CH3+H2O
��������б����£�
�Ҵ� | 1��2-�������� | ���� | �� | |
״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� | ����ɫҺ�� |
�ܶ�/g��cm-3 | 0.79 | 2.18 | 0.71 | 3.10 |
�е�/�� | 78.5 | 131.4 | 34.6 | 58.8 |
�۵�/�� | -114.3 | 9.79 | - 116.2 | -7.2 |
ˮ���� | ���� | ���� | �� | ���� |
(1)��ȫƿb��ʵ�����ж������á���һ���Լ��ʵ�������dװ�D�е����Ƿ�����������д����������ʱƿb�е�����_______________�����ʵ��ʱdװ�D�е��ܶ���������Ϊ���ܵ�ԭ���Ǣ�_______________����ȫƿb�������������Ǣ�_______________��
(2)����c��e�ж�ʢ��NaOH��Һ��c��NaOH��Һ��������_____________________��
(3)ijѧ��������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ����������������³������࣬���װ�D��������û�����⣬�Է������ܵ�ԭ��______________��______________(д����������)��
(4)��ȥ����������δ��Ӧ��Br2�����е���Ҫ����Ϊ___________��Ҫ��һ���ᴿ�����в����б������_____________ (����ĸ)��
A���ؽᾧ B������ C����ȡ D������
(5)ʵ����Ҳ���Գ�ȥdװ�D��ʢ��ˮ���ձ�����Ϊ����ˮֱ�Ӽ��뵽dװ�D���Թ��У����ʱ��ˮ����������ȴ1��2-��������������⣬��������������______��