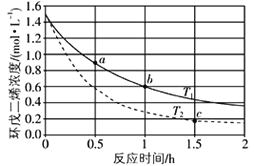
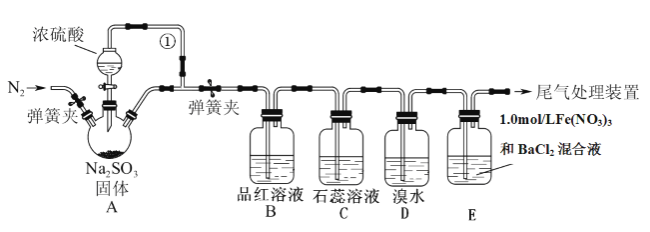
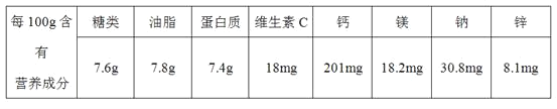
题目内容
【题目】Na2S溶液在空气中长期放置,与氧气反应会生成与过氧化钠的结构和化学性质相似的物质Na2S2,其溶液显黄色并有强碱性。
(1)写出该反应的化学方程式:___________________________________。并写出Na2S2的电子式___________该物质中所具有的化学键的类型_______________;该反应中的Na2S表现了_____________性,当生成1mol Na2S2时,转移电子数目为___________。
(2)在碱性溶液中,多硫化钠Na2Sx(x≥2),可被NaClO氧化为硫酸钠,而NaClO被还原为NaCl,若反应中Na2Sx与NaClO的物质的量之比为1:16,则X的值为________。
A.5 B.4 C.3 D.2
(3)常温下Na2S溶液显______(填酸性、中性或碱性),理由是(用离子方程式表示)__________________________。
(4)常温pH=10的0.1mol/LNaHS溶液中各离子浓度由大到小的顺序是_____________________________。
【答案】4Na2S + O2 + 2H2O → 4NaOH + 2Na2S2(或2Na2S + O2+2H2O→4NaOH+2S Na2S+S →Na2S2) ![]() 离子键和共价键 还原 2NA个 A 碱性 S2-+H2OHS-+OH- c(Na+)>c(HS -)>c(OH-)>c(H+)> c(S2-)
离子键和共价键 还原 2NA个 A 碱性 S2-+H2OHS-+OH- c(Na+)>c(HS -)>c(OH-)>c(H+)> c(S2-)
【解析】
Na2S2类似于过氧化钠;根据元素化合价的变化分析物质体现的性质;溶液的酸碱性是由盐类水解决定的;通过出现在前,不出现在后的原则分析溶液中的离子浓度关系,且伴随着水解平衡、电离平衡、水的电离进行具体分析。
(1) 在空气中长期放置会接触大量的氧气和水蒸气,故反应方程式为:4Na2S + O2 + 2H2O → 4NaOH + 2Na2S2,是离子化合物,电子式为:![]() ;中含共价键和离子键;硫元素化合价由负二价变成了负一价,体现了还原性;生成1mol Na2S2时,硫共失去2个电子,故转移电子数目为2NA;
;中含共价键和离子键;硫元素化合价由负二价变成了负一价,体现了还原性;生成1mol Na2S2时,硫共失去2个电子,故转移电子数目为2NA;
(2)多硫化钠Na2Sx被NaClO氧化为硫酸钠,而NaClO被还原为NaCl,反应中Na2Sx与NaClO的物质的量之比为1:16,由电子守恒可知![]() ,得x=5,故答案为:A;
,得x=5,故答案为:A;
(3)常温下Na2S溶液,硫离子发生水解:![]() ,故溶液显碱性;
,故溶液显碱性;
(4)常温pH=10的0.1mol/LNaHS溶液显碱性,硫氢根离子即水解又电离,但程度不同,水解:![]() ,电离:
,电离:![]() ,则是水解大于电离,故水解产物浓度大于电离产物浓度,且溶液中存在水的电离平衡,有离子关系:
,则是水解大于电离,故水解产物浓度大于电离产物浓度,且溶液中存在水的电离平衡,有离子关系:![]() 。
。

【题目】某次实验室制取乙酸丁酯所用原料为:7.4 mL1-丁醇、6.0 mL冰醋酸,1.0mL浓硫酸。
1-丁醇 | 冰醋酸 | |
密度(g/cm3) | 0.81 | 1.05 |
摩尔质量(g/mol) | 74 | 60 |
若制得乙酸丁酯(式量116)的质量为5.12 g,则以下正确的是
A.产率:约54.49%B.产率:约42.04%
C.转化率:冰醋酸小于1-丁醇D.转化率:冰醋酸大于1-丁醇