��Ŀ����
��16�֣����ƺ�̼����Ϊԭ�ϡ���������װ���Ʊ���������ƣ��Ʊ���Ӧ�ɱ�ʾΪ��
2Na2 S +Na2CO3 + 4SO2 3Na2S2O3 +CO2������Ҫ��ش����⣺
3Na2S2O3 +CO2������Ҫ��ش����⣺
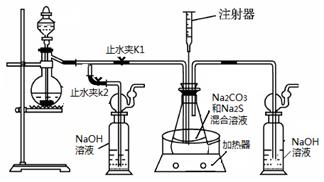
��1��ʵ��ʱ����K1���ر�K2�����ϴ��ƿ�з�����Ӧ�����ӷ���ʽ�� ��
��2����ƿ����ҺpHС��7ʱ�ᵼ�²�Ʒ�ֽ⣬���ʵ���������Ҫ������ƿ����Һ��pH��
�ٷ�Ӧ�����У���ƿ����ҺpH��________�����������С�����ֲ��䡱����
�ڲ�����ƿ����ҺpHʱ����ע������ȡ��Һ��Ʒ��ֱ�Ӵ���ƿ��ƿ��ȡ��������������⣬�����е��ŵ��� ��
����ʵ������в����ҺpH�ѽӽ���7����ʱӦ�ý��еIJ����� ��
��3����֪��2Na2 S2O3 +I2="2NaI+" Na2 S4O6��ʵ������������ش������ɼ����Na2 S2O3 ��5H2O���塣Ϊ�����䴿�ȣ�ȡ������Ʒmg����ˮ�ܽ���뼸�ε�����Һ����0��010mol/L��ˮ�ζ����յ�ʱ�����ĵ�ˮ��ҺvmL�������Ʒ������ ��
��4����ȡ��������Ƶ���һ�ַ�����ֱ�ӽ���ۺ��������ơ�ˮ��Ϲ�����ȡ��Ϊ̽����ȡ��������������������ҺpH����������Ũ�ȡ���Ӧ�¶ȡ������������������¶Ա�ʵ�飨ÿ��ʵ��ʱ��������������Ϊ63g����Ӧʱ��Ϊ30min����
��ʵ��1��2��Ŀ����̽����������Ũ�ȶ���������ת���ʵ�Ӱ�죬��a=
������Ҫ̽����ҺpH����Ӧ�¶ȡ������������������ת���ʵ�Ӱ�죬��ʵ��1��2�⣬���ٻ������ �ζԱ�ʵ��
��ʵ���������������ת���ʲ�������������ٵ�Ӱ�졣Ϊʲô��
��________________________________________ ___��
2Na2 S +Na2CO3 + 4SO2
 3Na2S2O3 +CO2������Ҫ��ش����⣺
3Na2S2O3 +CO2������Ҫ��ش����⣺
��1��ʵ��ʱ����K1���ر�K2�����ϴ��ƿ�з�����Ӧ�����ӷ���ʽ�� ��
��2����ƿ����ҺpHС��7ʱ�ᵼ�²�Ʒ�ֽ⣬���ʵ���������Ҫ������ƿ����Һ��pH��
�ٷ�Ӧ�����У���ƿ����ҺpH��________�����������С�����ֲ��䡱����
�ڲ�����ƿ����ҺpHʱ����ע������ȡ��Һ��Ʒ��ֱ�Ӵ���ƿ��ƿ��ȡ��������������⣬�����е��ŵ��� ��
����ʵ������в����ҺpH�ѽӽ���7����ʱӦ�ý��еIJ����� ��
��3����֪��2Na2 S2O3 +I2="2NaI+" Na2 S4O6��ʵ������������ش������ɼ����Na2 S2O3 ��5H2O���塣Ϊ�����䴿�ȣ�ȡ������Ʒmg����ˮ�ܽ���뼸�ε�����Һ����0��010mol/L��ˮ�ζ����յ�ʱ�����ĵ�ˮ��ҺvmL�������Ʒ������ ��
��4����ȡ��������Ƶ���һ�ַ�����ֱ�ӽ���ۺ��������ơ�ˮ��Ϲ�����ȡ��Ϊ̽����ȡ��������������������ҺpH����������Ũ�ȡ���Ӧ�¶ȡ������������������¶Ա�ʵ�飨ÿ��ʵ��ʱ��������������Ϊ63g����Ӧʱ��Ϊ30min����
| ʵ����� | ��ҺpH | ����������ˮ�������� | ��Ӧ�¶� | ������� | ��������ת���� |
| 1 | 10 | 1��5��1 | 100 | 18 | 80��7% |
| 2 | a | 1��1��1 | 100 | 18 | 94��6% |
������Ҫ̽����ҺpH����Ӧ�¶ȡ������������������ת���ʵ�Ӱ�죬��ʵ��1��2�⣬���ٻ������ �ζԱ�ʵ��
��ʵ���������������ת���ʲ�������������ٵ�Ӱ�졣Ϊʲô��
��________________________________________ ___��
��1��CO2+2OH-=CO32-+H2O SO2+2OH-=SO32-+H2O ��ÿ��1�֣�
��2���ټ�С �ڷ�ֹ����������Ⱦ���� �۹ر�K1����K2 ����2�֣���6�֣�
��3�� 0.496v /m��3�֣�
��4����10 ��1�֣� ��3 ��2�֣� ����Ϊ���壬��Ӱ��ƽ����ƶ���2�֣�
��2���ټ�С �ڷ�ֹ����������Ⱦ���� �۹ر�K1����K2 ����2�֣���6�֣�
��3�� 0.496v /m��3�֣�
��4����10 ��1�֣� ��3 ��2�֣� ����Ϊ���壬��Ӱ��ƽ����ƶ���2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 2SCl2
2SCl2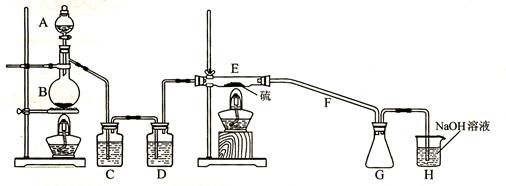
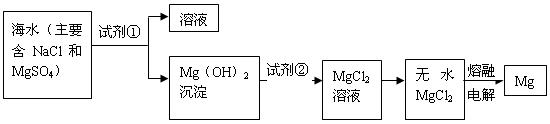
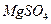 ת��Ϊ
ת��Ϊ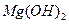 ���Լ��ٿ���ѡ�� ��Ҫʹ
���Լ��ٿ���ѡ�� ��Ҫʹ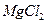 ������״̬�£�ͨ�������
������״̬�£�ͨ�������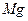 ��
��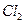 ���÷�Ӧ�Ļ�ѧ����ʽΪ��
���÷�Ӧ�Ļ�ѧ����ʽΪ��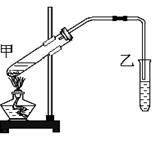
 �����ռ���и���
�����ռ���и���