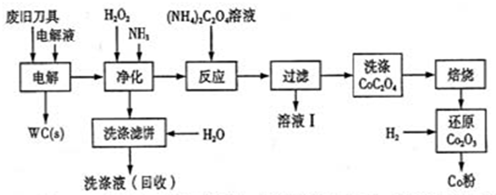
��Ŀ����
��������Դ�ı��⣬��ˮ�м����������е���ȻԪ�ء�������Դ��ѧ�����о��Ӻ�������ȡ��ѧ���ʵ�ѧ�ƣ������о��Ӻ�������ȡ����Ԫ���⣬���о��Ӻ�������ȡ��Ԫ�أ�Ũ��С��1mg/L����
��1�������к�����ߵ�±��Ԫ�������ڱ��е�λ��Ϊ ������ͬ���������ҵ���Ϊ�����Ԫ��ԭ�ӵĺ�������Ų�ʽΪ ��
��2��������Ԫ�غ���λ��ǰ�е�Ԫ���������ȡ��ơ�þ���������Ӱ뾶�Ӵ�С��˳��Ϊ �������ӷ��ű�ʾ���������γɵĻ��������ܷ���������� ���õ���ʽ��ʾ����
��3����Ԫ�����ں�ˮ����Ҫ��Be(OH)+��ʽ���ڣ�����������Ԫ�����ƣ�Ŀǰ�Ǵ��̱�ʯ����Ҫ�ɷ�Ϊ����������Be3Al2Si6O18������ȡ���������Ǻ��ա����ӡ������ȹ�ҵ���������ս�Խ������ϣ���˺�ˮ������Ϊ������Դ��ѧ�µ��о�������д����
�����������ε���������ʽ�Ļ�ѧʽ�� ��
��Be(OH)+��ǿ����Һ��Ӧ�����ӷ���ʽ�� ��
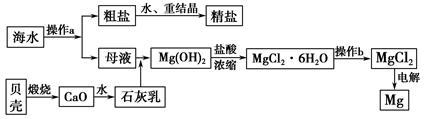
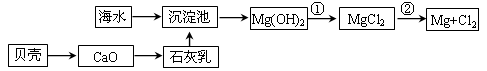
��4�����дӺ�������ȡ���ᴿ���ʵ�������ʵ�������У����������� ��ѡ���ţ���
a����ˮ���壺��ˮŨ��
 ������
������

 Һ��
Һ��
b����ˮ��þ����̲����
 ʯ����
ʯ����

 MgO
MgO þ
þ
c��������⣺��������
 ��Һ
��Һ �����л���Һ
�����л���Һ �⾧��
�⾧��
d�������ᴿ������

 ����
����

 ��Һ
��Һ
 ʳ�ξ���
ʳ�ξ���
������8�֣���1���������ڢ�A�� 1s22s22p63s23p4
��2��S2-��Cl-��O2-��Na+��Mg2+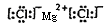
��3��3BeO��Al2O3��6SiO2 Be(OH)++3OH-��BeO22-+ 2H2O ��4��bd��2�֣�
���������������1�������к�����ߵ�±��Ԫ������Ԫ�أ���ԭ��������17�������ڱ��е�λ��Ϊ�������ڢ�A�壻����ͬ���������ҵ���Ϊ�����Ԫ����S��ԭ��������16�����ݹ���ԭ����֪Sԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p4��
��2�����Ӳ���Խ�����Ӱ뾶Խ���ں�������Ų���ͬ�������£����Ӱ뾶��ԭ���������������С�����������ȡ��ơ�þ���������Ӱ뾶�Ӵ�С��˳��ΪS2-��Cl-��O2-��Na+��Mg2+�������γɵĻ��������ܷ�����������Ȼ�þ���������Ӽ������ӻ���������ʽ��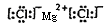 ��
��
��3����������������ʽ��ʾʱ�����ý�������������ǰ�棬Ȼ���Դ����ƣ����Ը������������εĻ�ѧʽBe3Al2Si6O18��֪������������ʽ�ɱ�ʾΪ3BeO��Al2O3��6SiO2��
��������Ԫ���Լ����������������Ԫ���Լ���������������ƣ����Ը�����������������������Һ��Ӧ�ķ���ʽ��֪��Be(OH)+��ǿ����Һ��Ӧ�����ӷ���ʽΪBe(OH)++3OH-��BeO22-+ 2H2O��
��4����������ͼ��֪��ac��ȷ����ҵ����Ȼ�þұ������þ�������ǵ������þ����Ϊ����þ���۵�̫�ߣ�b����ȷ��d���Ȼ�������������������Ba2�����ӣ����Եò����������Ȼ��ƣ�Ӧ���ȼ��Ȼ��������̼���ƣ����˺���������ữ��d����ȷ����ѡbd��
���㣺���麣ˮӦ�õ��й��жϣ�Ԫ�����ڱ��Ľṹ��Ԫ�������ɵ�Ӧ�õ�

 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д����г���������ұ��ԭ���в��������ǣ� ��
| | ���� | ұ��ԭ�� |
| A | Fe | 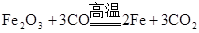 |
| B | Hg |  |
| C | Al | 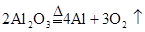 |
| D | Na |  |
(1)���з�Ӧԭ�������Ϲ�ҵұ������ʵ���������(����)��
A��2HgO 2Hg+O2�� 2Hg+O2�� | B��Fe3O4+4CO 3Fe+4CO2 3Fe+4CO2 |
C��2MgO 2Mg+O2�� 2Mg+O2�� | D��2Ag2O 4Ag+O2�� 4Ag+O2�� |