ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩAΓΔBΓΔCΓΔDΓΔEΓΔFΝυ÷÷‘ΣΥΊΨυΈΜ”Ύ÷ήΤΎ±μΒΡ«ΑΥΡ÷ήΤΎΘ§«“‘≠Ή”–ρ ΐ“ά¥Έ‘ω¥σΓΘ‘ΣΥΊA «‘≠Ή”ΑκΨΕΉν–ΓΒΡ‘ΣΥΊΘΜB‘ΣΥΊΜυΧ§‘≠Ή”ΒΡΚΥΆβΒγΉ”Ζ÷’ΦΥΡΗω‘≠Ή”ΙλΒάΘ®ΡήΦΕΘ©ΘΜD‘ΣΥΊ‘≠Ή”ΒΡ“―≥…Ε‘ΒγΉ”Ήή ΐ «Έ¥≥…Ε‘ΒγΉ”Ήή ΐΒΡ3±ΕΘΜE”κD¥Π”ΎΆ§“Μ÷ςΉεΘΜFΈΜ”Ύds«χΘ§«“‘≠Ή”ΒΡΉνΆβ≤ψ÷Μ”–1ΗωΒγΉ”ΓΘ
Θ®1Θ©E+άκΉ”ΒΡΒγΉ”≈≈≤Φ Ϋ « ΓΘ
Θ®2Θ©BΓΔCΓΔD‘ΣΥΊΒΡΒΎ“ΜΒγάκΡή”…¥σΒΫ–ΓΒΡΥ≥–ρ « ΓΘ
Θ®3Θ©BΓΔC‘ΣΥΊΒΡΡ≥–©«βΜ·ΈοΒΡΖ÷Ή”÷–ΨυΚ§”–18ΗωΒγΉ”Θ§‘ρBΒΡ’β÷÷«βΜ·ΈοΒΡΜ·―ß Ϋ « ΘΜBΓΔCΒΡ’β–©«βΜ·ΈοΒΡΖ–Βψœύ≤νΫœ¥σΒΡ÷ς“Σ‘≠“ρ « ΓΘ
Θ®4Θ©AΓΔBΓΔDΩ…–Έ≥…Ζ÷Ή” ΫΈΣA2BDΒΡΡ≥Μ·ΚœΈοΘ§‘ρΗΟΜ·ΚœΈοΖ÷Ή”÷–B‘≠Ή”ΒΡΙλΒά‘”Μ·άύ–Ά « ΘΜ1 molΗΟΖ÷Ή”÷–Κ§”–Π–ΦϋΒΡ ΐΡΩ « ΓΘ
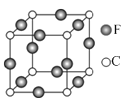
Θ®5Θ©CΓΔEΝΫ‘ΣΥΊ–Έ≥…ΒΡΡ≥Μ·ΚœΈοΒΡΨßΑϊΫαΙΙ»γΆΦΥυ ΨΘ§‘ρΗΟΜ·ΚœΈοΒΡΜ·―ß Ϋ « Θ§C‘≠Ή”ΒΡ≈δΈΜ ΐ « ΓΘ
ΓΨ¥πΑΗΓΩΘ®1Θ©1s22s22p63s23p63d10ΘϊΜρ[Ar] 3d10Θΐ
Θ®2Θ©NΘΨOΘΨC
Θ®3Θ©C2H6ΒΣΒΡ«βΜ·Έο(N2H4)Ζ÷Ή”Φδ¥φ‘Ύ«βΦϋ
Θ®4Θ©sp2(1Ζ÷) 1 mol(1Ζ÷)
Θ®5Θ©Cu3N(1Ζ÷) 6
ΓΨΫβΈωΓΩ ‘ΧβΖ÷ΈωΘΚ‘ΣΥΊA «‘≠Ή”ΑκΨΕΉν–ΓΒΡ‘ΣΥΊΘ§A «H‘ΣΥΊΘΜB‘ΣΥΊΜυΧ§‘≠Ή”ΒΡΚΥΆβΒγΉ”Ζ÷’ΦΥΡΗω‘≠Ή”ΙλΒάΘ®ΡήΦΕΘ©Θ§B «C‘ΣΥΊΘΜD‘ΣΥΊ‘≠Ή”ΒΡ“―≥…Ε‘ΒγΉ”Ήή ΐ «Έ¥≥…Ε‘ΒγΉ”Ήή ΐΒΡ3±ΕΘ§D «O‘ΣΥΊΘΜE”κD¥Π”ΎΆ§“Μ÷ςΉεΘ§E «P‘ΣΥΊΘΜFΈΜ”Ύds«χΘ§«“‘≠Ή”ΒΡΉνΆβ≤ψ÷Μ”–1ΗωΒγΉ”Θ§F «Cu‘ΣΥΊΓΘAΓΔBΓΔCΓΔDΓΔEΓΔF‘≠Ή”–ρ ΐ“ά¥Έ‘ω¥σΘ§C «N‘ΣΥΊΘΜΘ®1Θ©Cu+άκΉ”ΒΡΒγΉ”≈≈≤Φ Ϋ «1s22s22p63s23p63d10ΘΜΘ®2Θ©Ά§÷ήΤΎ‘ΣΥΊ¥”ΉσΒΫ”“ΒΎ“ΜΒγάκΡή÷πΫΞ‘ω¥σΘ§N‘≠Ή”pΙλΒάΈΣΑκ≥δ¬ζΉ¥Χ§Θ§ΒΎ“ΜΒγάκΡή¥σ”ΎO‘≠Ή”Θ§Υυ“‘CΓΔNΓΔO‘ΣΥΊΒΡΒΎ“ΜΒγάκΡή”…¥σΒΫ–ΓΒΡΥ≥–ρ «NΘΨOΘΨCΘΜΘ®3Θ©CΒΡ«βΜ·ΈοΒΡΖ÷Ή”÷–ΨυΚ§”–18ΗωΒγΉ”ΒΡΜ·ΚœΈο «C2H6ΘΜCΓΔNΒΡ’β–©«βΜ·ΈοΒΡΖ–Βψœύ≤νΫœ¥σΒΡ÷ς“Σ‘≠“ρ «ΒΣΒΡ«βΜ·Έο(N2H4)Ζ÷Ή”Φδ¥φ‘Ύ«βΦϋΘΜΘ®4Θ©ΦΉ»©Ζ÷Ή”÷–C‘≠Ή”ΒΡΙλΒά‘”Μ·άύ–Ά «sp2ΘΜΦΉ»©Ζ÷Ή”÷–Κ§”–1ΗωΥΪΦϋΘ§1 molΗΟΖ÷Ή”÷–1 molΠ–ΦϋΘΜΘ®5Θ©NΓΔCuΝΫ‘ΣΥΊ–Έ≥…ΒΡΡ≥Μ·ΚœΈοΒΡΨßΑϊΫαΙΙ»γ”“ΆΦΥυ ΨΘ§ΗυΨίΨυΧ·‘≠‘ρΘ§Ά≠‘≠Ή” ΐ![]() Θ§ΒΣ‘≠Ή” ΐ
Θ§ΒΣ‘≠Ή” ΐ![]() Θ§‘ρΗΟΜ·ΚœΈοΒΡΜ·―ß Ϋ «Cu3NΘ§άκN‘≠Ή”ΉνΫϋ«“ΨύάκœύΒ»ΒΡ‘≠Ή”Ι≤6ΗωΘ§N‘≠Ή”ΒΡ≈δΈΜ ΐ «6ΓΘ
Θ§‘ρΗΟΜ·ΚœΈοΒΡΜ·―ß Ϋ «Cu3NΘ§άκN‘≠Ή”ΉνΫϋ«“ΨύάκœύΒ»ΒΡ‘≠Ή”Ι≤6ΗωΘ§N‘≠Ή”ΒΡ≈δΈΜ ΐ «6ΓΘ