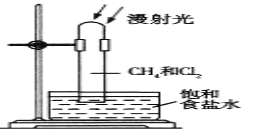
��Ŀ����
����Ŀ��������Ϊԭ�Ͻ����ۺ����ã�������߾���Ч�棬���ٶԻ�������Ⱦ����ͼ��ʾΪ��������ԭ����ȡ�����ʵ�ת�����̡�
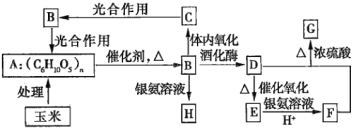
��֪��
��.G�Ǿ��й�����ζ��Һ�壻D����Ҫ�Ļ���ԭ�ϣ����ҿ��Դ�������������ȼ�ϡ�
��.![]() ��
��
��1��A������Ϊ________��B�ķ���ʽΪ________��F�Ľṹ��ʽΪ________��G�Ľṹ��ʽΪ________��
��2�����й���B��˵����ȷ����________������ţ���
a.1molB��ˮ������2molCH3CH2OH��2molCO2
b.������A���ɵ�B�Ƿ���л�ԭ�ԣ�����ˮ������Һ��ֱ�Ӽ������Ƶ�Cu(OH)2����Һ������
c.B���ܷ���������Ӧ
d.���ǵ�ˮ�����֮һΪB
��3����C��B������ת�������У�������ת����ʽΪ________��ת��Ϊ________�ܡ�
��4��D��E�Ļ�ѧ����ʽΪ________________��
��5��д��������F����͡�Ũ�����ڼ��������·�����Ӧ�Ļ�ѧ����ʽ��________________����Ӧ����Ϊ________��
���𰸡����� C6H12O6 CH3COOH CH3COOCH2CH3 d �� ��ѧ 2CH3CH2OH+O2![]() 2CH3CHO+2H2O 3CH3COOH+
2CH3CHO+2H2O 3CH3COOH+![]()
![]()
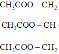 +3H2O ������Ӧ����ȡ����Ӧ��
+3H2O ������Ӧ����ȡ����Ӧ��
��������
����֪����D����Ҫ����ԭ���ҿ��Դ�������������ȼ�ϣ����ת��������D�ɱ�������֮�����������Һ��Ӧ��������֪DΪ�Ҵ�������D��E��F��D+F��G����G���й�����ζ����֪GΪ�����������ɴ˿�֪������ת������Ϊ����(A)�ڴ��������ȵ�������ˮ������������(B)��������������������ת�����̣��������ھƻ�ø�������·�����Ӧ�����Ҵ�(D)���Ҵ�������������Ӧ������ȩ(E)��������֪����![]() ��֪��FΪ���ᣬ�Ҵ���������Ũ���������·���������Ӧ������������(G)�������Ǻ�������Һ��Ӧ������������泥�H���������������ڱ������ɶ�����̼(C)��������̼ͨ���������ת��Ϊ�����ǣ�����ת���ɵ��ۡ��ݴ˽��з����жϡ�
��֪��FΪ���ᣬ�Ҵ���������Ũ���������·���������Ӧ������������(G)�������Ǻ�������Һ��Ӧ������������泥�H���������������ڱ������ɶ�����̼(C)��������̼ͨ���������ת��Ϊ�����ǣ�����ת���ɵ��ۡ��ݴ˽��з����жϡ�
��1���ɷ�����֪��A������Ϊ���ۣ������ǵķ���ʽΪC6H12O6������Ľṹ��ʽΪ��CH3COOH��GΪ������������ṹ��ʽΪ��CH3COOCH2CH3���ʴ�Ϊ�����ۣ�C6H12O6��CH3COOH��CH3COOCH2CH3��
��2��BΪ�����ǣ������к��еĹ�����Ϊ�ǻ���ȩ������
a�����������ڵ��ǣ�����ˮ�⣻a�����
b������ˮ����Ҫϡ������������ˮ������Һ�г����ԣ��������Ǻ����Ƶ�Cu(OH)2����Һ��Ҫ�ڼ��ԡ����������·�Ӧ��Ӧ�Ƚ�ˮ������Һ��������Ϊ���Ի������ټ������Ƶ�Cu(OH)2����Һ�����ȣ�b�����
c�������Ƿ����к���-OH���ܷ���������Ӧ��c�����
d���������ڶ��ǣ�ÿĦ������ˮ��������Ħ�����ǣ������Ǻ��ǡ�d����ȷ��
��ѡd��
��3��C��B�Ĺ���Ϊ������̼ͨ���������ת��Ϊ�����ǣ����е������仯Ϊ������ת��Ϊ��ѧ�ܣ���Ϊ���⣻��ѧ��
��4��D��E���Ҵ���������������ȩ�Ĺ��̣���Ӧ����ʽΪ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O����Ϊ��2CH3CH2OH+O2
2CH3CHO+2H2O������2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
��5��������ͣ�����������Ũ���ᡢ���ȵ������·���������Ӧ��3CH3COOH+![]()
![]()
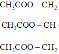 +3H2O����Ӧ����Ϊ��������Ӧ����ȡ����Ӧ������Ϊ��3CH3COOH+
+3H2O����Ӧ����Ϊ��������Ӧ����ȡ����Ӧ������Ϊ��3CH3COOH+![]()
![]()
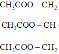 +3H2O��������Ӧ����ȡ����Ӧ����
+3H2O��������Ӧ����ȡ����Ӧ����

 �ο�������ϵ�д�
�ο�������ϵ�д� ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д�����Ŀ�����Ȼ�����(S2Cl2)��һ����Ҫ�Ļ���ԭ�ϣ��������������ı��������ȷ�ճ�������Ӳ�����ʡ��������Ͽ�֪S2Cl2�����������ʣ�
�������� | ���� | ɫ̬ | �ӷ��� | �۵� | �е� |
�綾 | ���ɫҺ�� | �ӷ� | ��76�� | 138�� | |
��ѧ���� | ��300��������ȫ�ֽ⣻��S2Cl2+Cl2 �������Ȼ�������Ӵ���������ȼ�յ�Σ�� �����Ȼ���ˮ�ֽ���ȣ��ų���ʴ������ | ||||
ʵ���ҿ�������������������110��140����Ӧ�Ƶ�S2Cl2��Ʒ����������ȡ����S2Cl2��װ�ã��ش���������:
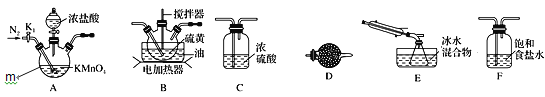
��1������m������Ϊ______��
��2��S2Cl2�ĵ���ʽΪ______��
��3��װ������˳��______��E��D��
��4��Ϊ�����S2Cl2�Ĵ��ȣ�ʵ��Ĺؼ��ǿ��ƺ��¶Ⱥ�________��
��5��S2Cl2��ˮǿ�ҷ�Ӧ�������������������һ������X��ʹƷ����Һ��ɫ�����Ⱥ��ָֻ�ԭ״ �ҷ�Ӧ������ֻ��һ��Ԫ�ػ��ϼ۷����仯��д���÷�Ӧ�Ļ�ѧ����ʽ_______��
��6��ijͬѧΪ�˲ⶨS2Cl2��ˮ��Ӧ�����ɵ�����X�ڻ�������е�����������������ʵ�鷽����
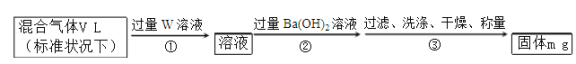
�û������������X���������Ϊ_____(�ú�V��m��ʽ�ӱ�ʾ)��
����Ŀ������a��e����ѧ��ѧʵ���г����ļ��ֶ���������
a.��Ͳ b.����ƿ c.�ζ��� d.������ƽ e.�¶ȼ�
��1������0���̶ȵ���___(����ĸ)��
��2�����в�����������___(����ĸ)��
A.��25 mL��ʽ�ζ�����ȡ20.00 mL NaHCO3��Һ
B.��������ƽȷ����10.20 g̼���ƹ���
C.��100 mL��Ͳ��ȡ3.2 mLŨ����
D.��240 mL����ƿ����240mL 1 mol��L��1������������Һ
��3��ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һ�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ����������������Ϊ___mL��

��4��ijѧ����������ʵ��ֱ��¼�й��������±���
�ζ����� | ��������������Һ�����/mL | 0.100 0 mol��L��1��������/mL | ||
�ζ�ǰ�̶� | �ζ���̶� | ��Һ��� | ||
��һ�� | 25.00 | 0.00 | 26.05 | 26.05 |
�ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
������ | 25.00 | 0.36 | 26.31 | 25.95 |
��ѡ�����к��������г�������������Һ���ʵ���Ũ�ȣ�c(NaOH)��___mol��L��1��
��5�����ڴ��������ʹ��������������������Һ��Ũ��ƫ�ߵ���___(����ĸ)��
A���ζ����յ�ʱ���ӵζ�����Һ�����
B����ʽ�ζ���������ˮϴ��������ȡ��25.00 mL�������Һע����ƿ���еζ�
C����ƿ�ô����Һ��ϴ
D���ζ�ʱ��ʽ�ζ�����������������ƿ��