��Ŀ����
����Ŀ����ͼ�Ƕ���(C4H10)�ѽ��ʵ�����̡����Ӻ�װ�ú�����е�ʵ������У��ټ�������װ�õ������Ԣ��ų�װ���еĿ����۸�D��Gװ�ü��ȵȡ�����G����װ���Լ�����̨����ʡ�ԣ�CuO�ܽ���������CO2��H2O��

��ش��������⣺
(1)�����ѽ�Ŀ��ܷ���ʽΪC4H10![]() CH4+C3H6��____________________��
CH4+C3H6��____________________��
(2)д������������ͭ��Ӧ�Ļ�ѧ����ʽ_____________________________��
(3)���Է�Ӧ��Eװ���еĻ����(��ˮ����),�ٰ���������ʵ�飺
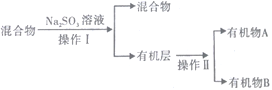
�ٷ��������͢�����Ʒֱ���___________��___________(����ĸ) ��
a������ b������ c����Һ d������
��Na2SO3��Һ��������(�����ӷ���ʽ��ʾ)_____________________________________________��
����֪A��̼ԭ��������B��̼ԭ��������д��B������______________________��
(4)�ٶ�������ȫ�ѽ⣬����D��Gװ���е���������ȫ��Ӧ����(E+F)װ�õ��������ȷ�Ӧǰ������0.49g��Gװ�õ������ȷ�Ӧ�������1.44��������ѽ�����м������������ʵ���֮��Ϊ___________��
���𰸡� C4H10![]() C2H6+C2H4 CH4��4CuO
C2H6+C2H4 CH4��4CuO ![]() 4Cu��CO2��2H2O c d SO32-+Br2+H2O�TSO42-+2H++2Br- 1��2-�������� 1��2
4Cu��CO2��2H2O c d SO32-+Br2+H2O�TSO42-+2H++2Br- 1��2-�������� 1��2
��������(1)����ԭ���غ㣬��ѭ������ɹ��ɣ�д�����ܷ����ķ�Ӧ��
(2) ������������,����������,���������ͭ��Ӧ���ɶ�����̼��ˮ��ͭ��
��3���ٻ�����к����塢ˮ�������,������������,�������Ʊ�����������������,ͬʱ����NaBr,�Ӷ���ȥ��,Ȼ����÷�Һ��������,���л�����з���õ��л���A���л���B��
��Na2SO3���л�ԭ�ԣ��ܹ�������������Ϊ�嵥�ʶ���ȥ�塣
����֪A��̼ԭ��������B��̼ԭ������˵��B��̼ԭ�Ӹ�����3��A��̼ԭ�Ӹ�����2��
(4) E��F���յ���ϩ��,G���ٵ�����������ͭ�е���Ԫ������,������ѽ���,������ϩ�����ʵ�������������ʵ������,����ͱ�ϩ�����ʵ������,�ٽ��ԭ���غ����������������ʵ���֮����
(1)�����ѽ�Ŀ��ܷ���ʽΪC4H10![]() CH4+C3H6�����黹���ܴ��ѻ�Ϊ��ϩ�����飺����ʽΪ. C4H10
CH4+C3H6�����黹���ܴ��ѻ�Ϊ��ϩ�����飺����ʽΪ. C4H10![]() C2H6+C2H4����ȷ����C4H10
C2H6+C2H4����ȷ����C4H10![]() C2H6+C2H4��
C2H6+C2H4��
(2) ������������,����������,���������ͭ��Ӧ���ɶ�����̼��ˮ��ͭ,��Ӧ����ʽΪ: CH4��4CuO ![]() 4Cu��CO2��2H2O����ȷ����CH4��4CuO
4Cu��CO2��2H2O����ȷ����CH4��4CuO ![]() 4Cu��CO2��2H2O��
4Cu��CO2��2H2O��
(3)�ٸ��ݷ�Ӧ���̿�֪����������������Һ�����ֲ㣬���Խ��з�Һ�������з��룻�����ֻ��ܵ��л���ֿ������Բ�������ķ������У���ȷ�𰸣�c��d��
��Na2SO3���л�ԭ�����ܹ�������������Ϊ�嵥�ʶ���ȥ�������ӷ���ʽ��SO32-+Br2+H2O�TSO42-+2H++2Br-����ȷ����SO32-+Br2+H2O�TSO42-+2H++2Br- ��
����֪A��̼ԭ��������B��̼ԭ������˵��B��̼ԭ�Ӹ�����3��A��̼ԭ�Ӹ�����2,��ϩ���巢���ӳ�����1��2-�������飬����AΪ 1��2-�������飻��ȷ�𰸣�1��2-�������顣
(4)������ѽ������ɵ���ϩ����������ʵ�����������ɵļ���ͱ�ϩ�����ʵ������,E��F���յ���ϩ��,G���ٵ�����������ͭ�е���Ԫ����������xΪC2H4�����ʵ���,yΪC3H6�����ʵ�����������ͼ�������ʵ����ֱ���x��y,28x+42y=0.49,����ͼ��������ͭ��Ӧ��Ҫ����ԭ�ӵ����ʵ���Ϊ:2(2x+y)+(6x+2y)/2=1.44/16,����ó�:x=0.01mol, y=0.005mol��������ѽ�����м������������ʵ���֮��Ϊ0.005��0.01=1:2����ȷ��:1:2��

����Ŀ����Դ����ϡ���Ϣ����Ϊ�ִ���ᷢչ������֧������ѧ����Դ����������ϵ��
(1)�±��е����ݱ�ʾ�ƻ�1mol��ѧ�������ĵ�����(�����ܣ���λΪkJ��mol-1)
��ѧ�� | H-H | Cl-Cl | H-Cl |
���� | 436 | 243 | 431 |
�����������Ϣ��֪��1mol������������������ȼ�������Ȼ�������ų�����___________��
(2)��Ȼ����һ����Ҫ�������Դ�ͻ���ԭ�ϣ�����Ҫ�ɷ�ΪCH4����CH4��������KOH��ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء��õ�صĸ�����ӦʽΪ___________��
(3)��ҵ����һ�ַ������� CO2������ȼ�ϼ״������Խ�CO2���Ϊ���������Ϊ1L���ܱ������У�����1molCO2��3molH2��һ�������·�����Ӧ��CO2+3H2![]() CH3OH(g)+H2O(g),���CO2(g)��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ��
CH3OH(g)+H2O(g),���CO2(g)��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ��

�ٴӷ�Ӧ��ʼ��ƽ�⣬CH3OH��ƽ����Ӧ����v(CH3OH)=___________��H2��ת���ʦ�(H2)=___________��
������ӦCO2+3H2![]() CH3OH(g)+H2O(g)�����ֲ�ͬ����µķ�Ӧ���ʷֱ�Ϊ��
CH3OH(g)+H2O(g)�����ֲ�ͬ����µķ�Ӧ���ʷֱ�Ϊ��
A��V(CO2)=0.15mol��L-1��min-1B��V(H2)=0.01mol��L-1��s-1
C��v(CH3OH)=0.2mol��L-1��min-1D��v(H2O)=0.45mol��L-1��min-1
�÷�Ӧ�����ɿ쵽����˳��Ϊ___________(����ĸ)��
(4)��ˮ��ѧ��Դ�����þ��зdz�������ǰ�����Ӻ�ˮ����ȡ��Ĺ�ҵ������ͼ��

�����̢����漰�����ӷ�Ӧ���£��������淽���������ʵ��Ļ�ѧ��������
��Br2+��CO32-=��BrO3-+��Br-+��CO2��______
����������������漰������ԭ��Ӧ����___________����
�۲�������ѻ������̬���壬������ֽ�֮ת��ɻ���̬���壬��Ŀ����___________��
����Ŀ���¶�ΪTʱ����4.0L�����ܱ������г���2.0mol PCl5 �� ������Ӧ��PCl5��g��PCl3��g��+Cl2��g������Ӧʱ�䣨t����������������ѹǿ��p�������ݼ�����
t/s | 0 | 50 | 150 | 250 | 350 |
��ѹǿp/100kPa | 100 | 116 | 119 | 120 | 120 |
��1������ѹǿp����ʼѹǿp0���㷴Ӧ��PCl5��ת���ʦ���PCl5���ı���ʽΪ��ƽ��ʱPCl5��ת����Ϊ���٣�
��2����Ӧ��ǰ50s��ƽ������v��PCl3��Ϊ���٣�
��3�����¶��µ�ƽ�ⳣ��Ϊ���٣�



