��Ŀ����
(12��)�����ڵ�����Ԫ��X��Y��Z��W��ԭ��ϵ����������Zԭ��������������X��Y��W����ԭ�ӵ�����������֮�ͣ�Z��X��Y��W����Ԫ���γ�ԭ�Ӹ���֮��Ϊ1��1�Ļ�����ֱ���A��B��C�����л�����C�ڿ��������ױ��ʣ���ش�
��1��д��Z��ԭ�ӽṹʾ��ͼ______________________________��
��2��д��������YZ2����ʽ��______________�ռ乹��Ϊ��_______________��
д��������C�ĵ���ʽ��_____________��ѧ�������У�_____________��
��3��д��������C�ڿ����б��ʵĻ�ѧ����ʽ��____________________
��1��д��Z��ԭ�ӽṹʾ��ͼ______________________________��
��2��д��������YZ2����ʽ��______________�ռ乹��Ϊ��_______________��
д��������C�ĵ���ʽ��_____________��ѧ�������У�_____________��
��3��д��������C�ڿ����б��ʵĻ�ѧ����ʽ��____________________
(1) 
(2) ;ֱ����
;ֱ����
(3) �����Ӽ����ۼ�
�����Ӽ����ۼ�
(4)2Na2O2 + 2CO2 ="==" 2Na2CO3 + O2 2Na2O2 + 2H2O ="==" 4NaOH + O2��

(2)
 ;ֱ����
;ֱ����(3)
 �����Ӽ����ۼ�
�����Ӽ����ۼ�(4)2Na2O2 + 2CO2 ="==" 2Na2CO3 + O2 2Na2O2 + 2H2O ="==" 4NaOH + O2��
�����ƶ�Ԫ�صĹؼ�����Ϥ����Ԫ�ؼ��仯����Ļ�ѧʽ����Ŀ���й�Z����Ϣ��࣬�ر��ǡ�Z��X��Y��W����Ԫ���γ�ԭ�Ӹ���֮��Ϊ1��1�Ļ�������ɲ²�AΪ��Ԫ�أ�XΪ�⣬WΪNa������������Ϣ��֪YΪ̼Ԫ�أ�
��1������ԭ�ӽṹʾ��ͼΪ��
��2��CO2Ϊֱ�����ۻ����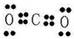 ����Na2O2 Ϊ���зǼ��Լ��������ͻ����
����Na2O2 Ϊ���зǼ��Լ��������ͻ����
��3��Na2O2 ��������е�ˮ��CO2��Ӧ��2Na2O2 + 2CO2 ="==" 2Na2CO3 + O2 2Na2O2 + 2H2O ="==" 4NaOH + O2��
��1������ԭ�ӽṹʾ��ͼΪ��

��2��CO2Ϊֱ�����ۻ����
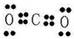 ����Na2O2 Ϊ���зǼ��Լ��������ͻ����
����Na2O2 Ϊ���зǼ��Լ��������ͻ����
��3��Na2O2 ��������е�ˮ��CO2��Ӧ��2Na2O2 + 2CO2 ="==" 2Na2CO3 + O2 2Na2O2 + 2H2O ="==" 4NaOH + O2��

��ϰ��ϵ�д�
 �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д� �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�
�����Ŀ
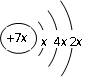 �� b��c�γɻ�����ĵ���ʽΪ
�� b��c�γɻ�����ĵ���ʽΪ 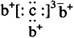 �����бȽ�����ȷ���ǣ� ��
�����бȽ�����ȷ���ǣ� ��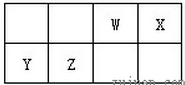
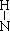 ��H
��H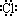 ]
]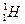 ��
�� ��
�� ��
��