��Ŀ����
17����1������AXn-������18�����ӣ������ĺ˵����Ϊ18-n������������ΪA-18+n������1H216O��2H217O�������������У�����2��Ԫ�أ�4�ֺ��أ�
��2����A��B��C����Ԫ�أ���֪AԪ��ԭ�ӵ�K���M���������ͬ��BԪ��ԭ�ӵ�L���K���������5����CԪ�ص�+3�������Ӻ���ԭ�Ӿ�����ͬ�ĵ�������Cԭ�ӵ�����������������1����
��д��A��ԭ�ӽṹʾ��ͼ
 ��Ԫ��C��ԭ����ɷ�����2713Al��
��Ԫ��C��ԭ����ɷ�����2713Al����A��B��ɵĻ�����Ļ�ѧʽMgF2��
��3��14.7gH2SO4����������ԭ������2.24 L ����״���£�NH3������ԭ������ȣ�
��4������״���µ� a L HCl��������1000gˮ�У��õ������ܶ�Ϊb g/cm3�������������ʵ���Ũ����$\frac{1000ab}{22400+36.5a}$mol/L��
���� ��1���ٺ˵����=�����������������=������-������ɣ�������=������-��������
����1H216O��2H217O�������������У�����H��O����Ԫ�أ���1H��16O��2H��17O���ֺ��أ�
��2����A��B��C����Ԫ�أ�AԪ��ԭ�ӵ�K���M���������ͬ����AΪMg��BԪ��ԭ�ӵ�L���K���������5����L�������Ϊ7����BΪFԪ�أ�CԪ�ص�+3�������Ӻ���ԭ�Ӿ�����ͬ�ĵ�������Cԭ�ӵ�����������������1������CΪAl��
��3������n=$\frac{m}{M}$����14.7gH2SO4���ӵ����ʵ�����������ԭ������ȼ��㰱�����ʵ������ٸ���V=nVm���㰱�������
��4������n=$\frac{V}{{V}_{m}}$����HCl���ʵ���������m=nM����HCl��������Һ����=ˮ������+HCl����������m=$\frac{m}{��}$������Һ������ٸ���c=$\frac{m}{V}$������Һ���ʵ���Ũ�ȣ�
��� �⣺��1������AXn-������18�����ӣ������ĺ˵����Ϊ18-n������������ΪA-��18-n��=A-18+n��
�ʴ�Ϊ��18-n��A-18+n��
���������=������-������ɣ�������=������-��������
����1H216O��2H217O�������������У�����H��O����Ԫ�أ���1H��16O��2H��17O���ֺ��أ�
��2����A��B��C����Ԫ�أ�AԪ��ԭ�ӵ�K���M���������ͬ����AΪMg��BԪ��ԭ�ӵ�L���K���������5����L�������Ϊ7����BΪFԪ�أ�CԪ�ص�+3�������Ӻ���ԭ�Ӿ�����ͬ�ĵ�������Cԭ�ӵ�����������������1������CΪAl��
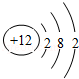
��AΪMgԪ�أ�ԭ�ӽṹʾ��ͼΪ�� ��CԪ�ص�+3�������Ӻ���ԭ�Ӿ�����ͬ�ĵ�������Cԭ�ӵ�����������������1������CΪAl��ԭ�ӷ���Ϊ��2713Al��
��CԪ�ص�+3�������Ӻ���ԭ�Ӿ�����ͬ�ĵ�������Cԭ�ӵ�����������������1������CΪAl��ԭ�ӷ���Ϊ��2713Al��
�ʴ�Ϊ�� ��2713Al��
��2713Al��
��A��B��ɵĻ�����Ļ�ѧʽΪMgF2���ʴ�Ϊ��MgF2��
��3��14.7gH2SO4���ӵ����ʵ���Ϊ$\frac{14.7g}{98g/mol}$=0.15mol����֮���������ԭ����Ŀ�İ������ʵ���Ϊ$\frac{0.15mol��2}{3}$=0.1mol������°������Ϊ0.1mol��22.4L/mol=2.24L��
��2.24��
��4������״���µ� a L HCl��������ʵ���Ϊ$\frac{aL}{22.4L/mol}$=$\frac{a}{22.4}$mol��������Ϊ$\frac{a}{22.4}$mol��36.5g/mol=$\frac{36.5a}{22.4}$g����Һ����Ϊ��1000+$\frac{36.5a}{22.4}$��g����Һ���=$\frac{��1000+\frac{36.5a}{22.4}��g}{1000bg/L}$=$\frac{22400+36.5a}{22400b}$L�������������ʵ���Ũ����$\frac{\frac{a}{22.4}mol}{\frac{22400+36.5a}{22400b}L}$=$\frac{1000ab}{22400+36.5a}$mol/L��
�ʴ�Ϊ��$\frac{1000ab}{22400+36.5a}$mol/L��
���� ���⿼��ṹ����λ�ù�ϵӦ�á�ԭ�ӹ��ɡ����ʵ����йؼ��㣬ע��Ի���֪ʶ���������գ�

| A�� | �û���Ӧһ����������ԭ��Ӧ | |
| B�� | ������̼�Ƿǵ���� | |
| C�� | �������Ķ�������Ͱ�����ǰ������� | |
| D�� | �������Թܡ����������þƾ���ֱ�Ӽ��� |
| A�� | ���ᱵ������ˮ����ˮ��Һ�в��ܵ��磬�������ᱵ���ǵ���� | |
| B�� | ǿ�������ˮ��Һ�еĵ�����һ�����������ǿ | |
| C�� | ������������ˮ�ܵ��磬�������������ǵ���� | |
| D�� | ���ᡢ������������������ƶ���ǿ����� |
| A�� | 0.1 mol•L-1 CH3COONa��Һ��0.1 mol•L-1 HCl��Һ�������ϣ�c��Na+��=c��Cl-����c��CH3COO-����c��H+����c��OH-�� | |
| B�� | 0.1 mol•L-1 NH4Cl��Һ��0.1 mol•L-1��ˮ�������ϣ�pH��7����c��NH3•H2O����c��NH${\;}_{4}^{+}$����c��Cl-����c��OH-�� | |
| C�� | 0.1 mol•L-1 Na2CO3��Һ��0.1 mol•L-1 NaHCO3��Һ�������ϣ�3c��Na+��=2c��CO${\;}_{3}^{2-}$��+2c��HCO${\;}_{3}^{-}$��+2c��H2CO3�� | |
| D�� | 0.1 mol•L-1 Na2C2O4��Һ��0.1 mol•L-1 HCl��Һ�������ϣ�H2C2O4Ϊ��Ԫ���ᣩ��2c��C2O${\;}_{4}^{2-}$��+c��HC2O${\;}_{4}^{-}$��+c��OH-��=c��H+��+c��Cl-�� |
������֪��448��ʱ����ӦH2��g��+I2��g���T2HI��g����ƽ�ⳣ��K1Ϊ49������¶��·�Ӧ2HI��g���TH2��g��+I2��g����ƽ�ⳣ��K2Ϊ$\frac{1}{49}$��
������һ��������ܱ������У��������»�ѧ��Ӧ��CO2��g��+H2��g���TCO��g��+H2O��g�����仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���ʾ��
| t/�� | 700 | 800 | 830 | 1000 | 1200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
��1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK=$\frac{c��CO����c��{H}_{2}O��}{c��C{O}_{2}����c��{H}_{2}��}$��
��2���÷�ӦΪ���ȷ�Ӧ������ȡ����ȡ�����������ѹǿʱ��ƽ�ⲻ���������������ƶ�
��3�����жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������BC
A��������ѹǿ���� B�����������C��CO������
C��V��H2����=V��H2O���� D��c��CO2��=c��CO��