��Ŀ����
���ʴ��ֳ��Ҷ����ѣ�����ʽΪC4H10O3��HO-CH2-CH2-O-CH2-CH2-OH�������ʴ���һ����Ҫ�Ļ���ԭ�ϣ�������ȡ�ᡢ�������ȣ���;ʮ�ֹ㷺�����ʴ�һ��ĺϳ�·�����£�
���̢� Br2 ������ ��Ӧ��
��1�����̢���ʯ�ͼӹ��г��ò��裬������Ϊ ��������B������C�ķ�Ӧ�������� ���÷�Ӧ���� ����д��Ӧ���ͣ�������B������C�Ĺ�������������Ʋ��û���������E��E�����ڽ������ид��B��������E�Ļ�ѧ����ʽ ��
��2��д�������ϳ�·���е�����A��B��C�Ľṹ��ʽ��
A ��B ��C
��3����Ӧ��Ļ�ѧ����ʽΪ�� ��
Br2��
��1���ѽ⣨1�֣���NaOHˮ��Һ��1�֣���ȡ����ˮ�⣩��Ӧ��1�֣���
CH2BrCH2Br + 2NaOH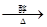 HC��CH��+ 2 NaBr ��1�֣�
HC��CH��+ 2 NaBr ��1�֣�
��2��A��CH2=CH2��B��Br-CH2-CH2-Br��C��HO-CH2-CH2-OH��1��1��3�֣�
��3��2HO-CH2-CH2-OH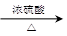 2HO-CH2-CH2-O-CH2-CH2-OH+H2O��1�֣�
2HO-CH2-CH2-O-CH2-CH2-OH+H2O��1�֣�
���������������1��ʯ�͡�����A �� ����B ����ˮ��Ӧ��˵����˫��������Ҫ�ƶ��ʴ�����̼�����٣�ʯ�͡�����A��������̷������ѽⷴӦ���ѽ�������ѻ�������С����ϩ����B��C��BΪ�������C
Ϊ���� ��NaOHˮ��Һ�з�����ˮ�ⷴӦ����ȡ����Ӧ�������и�����Ȳ�������ʡ�
CH2BrCH2Br + 2NaOH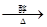 HC��CH��+ 2 NaBr
HC��CH��+ 2 NaBr
��2���ɺϳ�·�����ƶ��ʴ�:2HO-CH2-CH2-O-CH2-CH2-OH��HO-CH2-CH2-OH��Br-CH2-CH2-Br��CH2=CH2
���ԣ�A��CH2=CH2��B��Br-CH2-CH2-Br��C��HO-CH2-CH2-OH
���㣺�������л��ƶ�Ϊ�����������л�����������ʵ�֪ʶ��

��֪ij��ȼ�Ϻ���̼���⡢������Ԫ�ء�Ϊ�˲ⶨ����ȼ����̼��������Ԫ�ص������ȣ��ɽ���̬ȼ�Ϸ���������������ȼ�գ���ʹ����������ȫ��ͨ����ͼ��ʾ��װ�ã��õ����±����е�ʵ����(���������������ȫ������)��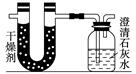
| | ʵ��ǰ | ʵ��� |
| (�������U�ι�)������ | 101.1 g | 102.9 g |
| (ʯ��ˮ�����ƿ)������ | 312.0 g | 314.2 g |
����ʵ��������
(1)ʵ����Ϻ���������ˮ������Ϊ________ g��
������ƿ������һ�����Σ�������Ϊ________ g��
(2)���ɵ�ˮ����Ԫ�ص�����Ϊ________ g��
(3)���ɵĶ�����̼��̼Ԫ�ص�����Ϊ________ g��
(4)��ȼ����̼Ԫ������Ԫ�ص�������Ϊ________��
(5)��֪���ִ���ÿ�������к���һ����ԭ�ӣ���ô��ķ���ʽΪ__________���ṹ��ʽΪ_______________________________��

 RCHO
RCHO


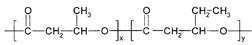 ����һ�ֿɽ���ĸ߷��Ӳ��ϡ������Ʒ������ʳƷ��װ����ױƷ��ҽҩ��������ũҵ����ҵ�������ɻ�Ϊͬϵ���M��N����Ϊ������������ԭ�Ͼ�����·�ߺϳɣ�
����һ�ֿɽ���ĸ߷��Ӳ��ϡ������Ʒ������ʳƷ��װ����ױƷ��ҽҩ��������ũҵ����ҵ�������ɻ�Ϊͬϵ���M��N����Ϊ������������ԭ�Ͼ�����·�ߺϳɣ�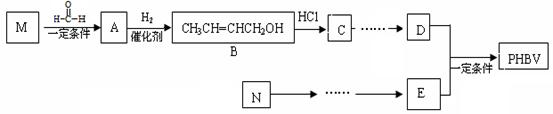
 CH3CHO
CH3CHO CH3COOH
CH3COOH

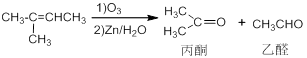
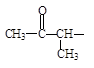 �������������ͬ���칹
�������������ͬ���칹 ����
����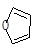 ��Ҳ���Է�����Diels-Alder��Ӧ�����û�ѧ��Ӧ����ʽΪ��
��Ҳ���Է�����Diels-Alder��Ӧ�����û�ѧ��Ӧ����ʽΪ��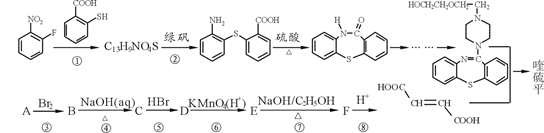
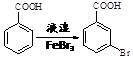 �����
��д����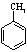 Ϊԭ���Ʊ�
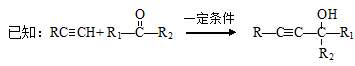
Ϊԭ���Ʊ� �ĺϳ�·������ͼ�����Լ���ѡ�������ñ����е������Ϣ�����ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�����Լ���ѡ�������ñ����е������Ϣ�����ϳ�·������ͼʾ�����£�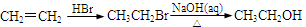 ��
��