��Ŀ����
ijУѧ����ѧʵ��С�飬Ϊ��֤�ǽ���Ԫ���ȵ�������ǿ����͵��������һ��ʵ��װ�ã������ּг�װ������ȥ��
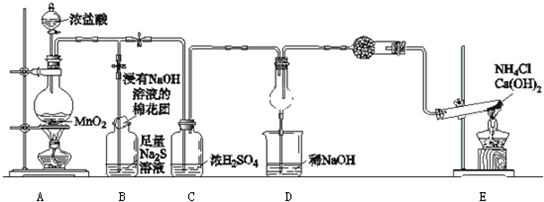
��1��д��A�з�Ӧ�����ӷ���ʽ______��
��2��B�г��ֻ�ɫ��������������������ӷ���ʽ______��
��3���Դ�ԭ�ӽṹ�ǶȽ����ȵ������Դ������ԭ��______��
��4��D�и�����г��ֵ�����ѧ����ʽ______��
��5����ͬѧ��ΪD�е�������˵���ȵ������Դ��ڵ�����Ҫ��C֮ǰ��װϴ��װ�ã��뻭����װ��ͼ______����ע��ʢװ�Լ�����
��6������ʲô������֤��������Cl2��S����һ�������ʵ˵��______��

��1��д��A�з�Ӧ�����ӷ���ʽ______��
��2��B�г��ֻ�ɫ��������������������ӷ���ʽ______��
��3���Դ�ԭ�ӽṹ�ǶȽ����ȵ������Դ������ԭ��______��
��4��D�и�����г��ֵ�����ѧ����ʽ______��
��5����ͬѧ��ΪD�е�������˵���ȵ������Դ��ڵ�����Ҫ��C֮ǰ��װϴ��װ�ã��뻭����װ��ͼ______����ע��ʢװ�Լ�����
��6������ʲô������֤��������Cl2��S����һ�������ʵ˵��______��
��1����Ӧװ��AΪŨ����Ͷ������̹����Ʊ�������װ�ã������ӷ���ʽΪ��MnO2+4H++2Cl
Mn2++Cl2��+2H2O��
�ʴ�Ϊ��MnO2+4H++2Cl
Mn2++Cl2��+2H2O��
��2����Ӧ�Ʊ�������ͨ��B֮��B�г��ֻ�ɫ��������˵��������������Һ�е�����������ΪS���ʣ����ӷ���ʽΪCl2+S2-=S��+2Cl-��
�ʴ�Ϊ��Cl2+S2-=S��+2Cl-��
��3����ԭ���������7�����ӣ���ԭ���������6�����ӣ��������Ӷࣨ��Ϊ8���õ���������ǿ���õ�������ǿ��������ǿ��
�ʴ�Ϊ����ԭ����������������ԭ�Ӷ�һ����Cl�õ�������ǿ����Cl2�����Դ���S��
��4��ͨEװ�����Ʊ������������ܰѰ����������ɵ������Ȼ��⣬���ɵ��Ȼ���Ͱ�����������Ȼ�泥��Ӷ�ð���̣����ʵ��������Dz����������̣���Ӧ�ķ���ʽ��8NH3+3Cl2=6NH4Cl+N2��
�ʴ�Ϊ�������������̣�8NH3+3Cl2=6NH4Cl+N2��
��5�������ӷ����������������к����Ȼ������壬�Ȼ���Ҳ��ֱ�ӺͰ������ð���̣�������Ҫ��C֮ǰ����һʢ�б���ʳ��ˮ��װ���Գ�ȥ�Ȼ������壬װ��ͼΪ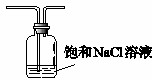 ��
��
�ʴ�Ϊ�� ��
��
��6���ǽ�����ǿ���Ƚϵ�һ������ǣ����ɶ�Ӧ�⻯����ȶ����жϣ��⻯��Խ�ȶ����ǽ�����Խǿ����������������Ӧˮ������������жϣ�����Խǿ���ǽ���Խǿ��������Ԫ��֮�⣩���۷ǽ�����������пɱ�۽����ķ�Ӧ�������ɸ۽���������ģ���ǽ�����ǿ���ݴ˿�����֤��
�ʴ�Ϊ������һ��2Fe+3Cl2
2FeCl3��Fe+S
FeS��
����������ͬ�¶�����ͬ״̬��HCl���ȶ��Դ���H2S��
������������������Ӧ��ˮ���������HClO4 ����H2SO4��
| ||
�ʴ�Ϊ��MnO2+4H++2Cl
| ||
��2����Ӧ�Ʊ�������ͨ��B֮��B�г��ֻ�ɫ��������˵��������������Һ�е�����������ΪS���ʣ����ӷ���ʽΪCl2+S2-=S��+2Cl-��
�ʴ�Ϊ��Cl2+S2-=S��+2Cl-��
��3����ԭ���������7�����ӣ���ԭ���������6�����ӣ��������Ӷࣨ��Ϊ8���õ���������ǿ���õ�������ǿ��������ǿ��
�ʴ�Ϊ����ԭ����������������ԭ�Ӷ�һ����Cl�õ�������ǿ����Cl2�����Դ���S��
��4��ͨEװ�����Ʊ������������ܰѰ����������ɵ������Ȼ��⣬���ɵ��Ȼ���Ͱ�����������Ȼ�泥��Ӷ�ð���̣����ʵ��������Dz����������̣���Ӧ�ķ���ʽ��8NH3+3Cl2=6NH4Cl+N2��
�ʴ�Ϊ�������������̣�8NH3+3Cl2=6NH4Cl+N2��
��5�������ӷ����������������к����Ȼ������壬�Ȼ���Ҳ��ֱ�ӺͰ������ð���̣�������Ҫ��C֮ǰ����һʢ�б���ʳ��ˮ��װ���Գ�ȥ�Ȼ������壬װ��ͼΪ
 ��
���ʴ�Ϊ��
 ��
����6���ǽ�����ǿ���Ƚϵ�һ������ǣ����ɶ�Ӧ�⻯����ȶ����жϣ��⻯��Խ�ȶ����ǽ�����Խǿ����������������Ӧˮ������������жϣ�����Խǿ���ǽ���Խǿ��������Ԫ��֮�⣩���۷ǽ�����������пɱ�۽����ķ�Ӧ�������ɸ۽���������ģ���ǽ�����ǿ���ݴ˿�����֤��
�ʴ�Ϊ������һ��2Fe+3Cl2
| ||
| ||
����������ͬ�¶�����ͬ״̬��HCl���ȶ��Դ���H2S��
������������������Ӧ��ˮ���������HClO4 ����H2SO4��

��ϰ��ϵ�д�
�����Ŀ
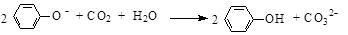
 2Cu����O2����4H��
2Cu����O2����4H��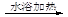
 Mn2��+2Cl��+Cl2��+2H2O
Mn2��+2Cl��+Cl2��+2H2O